Exam 16: Acids and Bases
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Calculate the pH of a solution containing 0.20 g of NaOH in 2.000L of solution.
(Short Answer)
4.8/5  (27)
(27)
Identify the conjugate base of HPO42- in the reaction HCO3- + HPO42-  H2CO3 + PO43-
H2CO3 + PO43-
(Multiple Choice)
4.8/5  (41)
(41)
Butyric acid is responsible for the odor in rancid butter. A solution of 0.25 M butyric acid has a pH of 2.71. What is the Ka for the acid?
(Multiple Choice)
4.8/5  (33)
(33)
Give the two factors that influence the extent to which an acid undergoes ionization.
(Essay)
4.9/5  (35)
(35)
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 mmol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 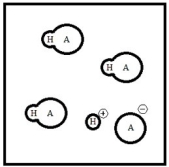 What is the percent ionization of HA?
What is the percent ionization of HA?
(Multiple Choice)
4.8/5  (35)
(35)
For H3PO4, Ka1 = 7.3 × 10-3, Ka2 = 6.2 × 10-6, and Ka3 = 4.8 × 10-13. A 0.10 M aqueous solution of NaH2PO4 therefore would be ________.
(Multiple Choice)
4.9/5  (31)
(31)
A solution is prepared by adding 0.10 mol of lithium nitrate, LiNO3, to 1.00 L of water. Which statement about the solution is correct?
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following is the correct equilibrium expression for the autoionization of water?
(Multiple Choice)
4.9/5  (33)
(33)
Hydroxylamine, HONH2, readily forms salts such as hydroxylamine hydrochloride which are used as antioxidants in soaps. Hydroxylamine has Kb = 9.1 × 10-9. What is the pH of a 0.025 M HONH2 solution?
(Multiple Choice)
4.9/5  (40)
(40)
A solution is prepared by adding 0.10 mol of potassium chloride, KCl, to 1.00 L of water. Which statement about the solution is correct?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 121 - 137 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)