Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
From the following list of aqueous solutions and water, select the one with the highest freezing point.
(Multiple Choice)
4.8/5  (44)
(44)
Cinnamaldehyde (132.16 g/mol) is used as a flavoring agent. What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.57°C? (For ethanol, Kb = 1.22°C/m; boiling point of pure ethanol = 78.37°C)
(Multiple Choice)
4.9/5  (39)
(39)
________ ________ are properties that depend on the number of particles dissolved.
(Short Answer)
4.9/5  (36)
(36)
Human blood has a molar concentration of solutes of 0.30 M. What is the osmotic pressure of blood at 25°C? (R = 0.08206 L • atm/K • mol)
(Multiple Choice)
4.9/5  (35)
(35)
What is the name given to the process when solvent molecules surround the solute molecules?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following compounds would be most likely to dissolve in CCl4?
(Multiple Choice)
4.9/5  (30)
(30)
Below is a representation of a sealed chamber containing pure liquid water in equilibrium with water vapor. The circles represent water vapor. 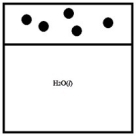 If a nonvolatile solute is added to the water, which best represents the new system at equilibrium?
If a nonvolatile solute is added to the water, which best represents the new system at equilibrium?
(Multiple Choice)
4.9/5  (34)
(34)
What is the name given to a solution that contains more solute than it has the capacity to dissolve?
(Multiple Choice)
4.9/5  (42)
(42)
________ is the process used to stabilize a colloid that would otherwise not stay dispersed.
(Short Answer)
4.8/5  (28)
(28)
Which process defines how molecular compounds form ions upon dissolution?
(Multiple Choice)
4.8/5  (36)
(36)
Raoult's Law relates the vapor pressure of the solvent above the solution to its mole fraction in the solution so that Raoult's law works best when applied to ________ (concentrated or dilute) solutions.
(Short Answer)
4.7/5  (33)
(33)
When sodium chloride dissolves in water, what stabilizes the ions in solution?
(Short Answer)
4.7/5  (34)
(34)
What is the osmotic pressure of a solution that contains 13.7 g of propyl alcohol (C3H7OH) dissolved in enough water to make 500. mL of solution at 27°C? (R = 0.08206 L • atm/K • mol)
(Multiple Choice)
4.9/5  (34)
(34)
Automobile radiators usually carry a sticker that indicates that they must contain an antifreeze solution for proper operation, winter or summer. Why should you not use ordinary water during the summer?
(Essay)
4.9/5  (36)
(36)
The distinguishing characteristic of all nonelectrolyte solutions is that they
(Multiple Choice)
4.9/5  (37)
(37)
Below is a diagram representing a solvent A(l) in a 1-L beaker, and a solute X dissolved in the solvent. Solvent A has a density of 0.8 g/mL, and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol. Each circle of X represents 1 mol of X. Assume that the solute addition does not significantly change the volume of liquid in the beaker. 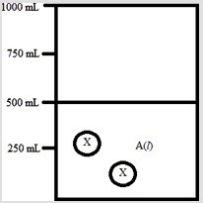 What is the molality of the solute X in this solution?
What is the molality of the solute X in this solution?
(Multiple Choice)
4.7/5  (46)
(46)
The density of a 20.3 M CH3OH (methanol) solution is 0.858 g/mL. What is the molality of this solution? H2O is the solvent.
(Multiple Choice)
4.9/5  (27)
(27)
Hexachlorophene is used as a disinfectant in germicidal soaps. What mass of hexachlorophene (406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? (For chloroform, Kb = 3.63°C/m; boiling point of pure chloroform = 61.20°C)
(Multiple Choice)
4.8/5  (42)
(42)
Showing 21 - 40 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)