Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
What is the freezing point of a solution prepared from 50.0 g ethylene glycol (C2H6O2) and 85.0 g H2O? (For water, Kf = 1.86°C/m)
(Multiple Choice)
4.8/5  (38)
(38)
Which substance is present in the largest proportion in a solution?
(Multiple Choice)
4.9/5  (36)
(36)
Diethyl ether has a vapor pressure of 400.0 torr at 18°C. When a sample of benzoic acid is dissolved in ether, the vapor pressure of the solution is 342 torr. What is the mole fraction of benzoic acid in the solution?
(Multiple Choice)
4.8/5  (35)
(35)
What is the freezing point of a solution made from 22.0 g of octane (C8H18) dissolved in 148.0 g of benzene? (For benzene, freezing point = 5.50°C; Kf = 5.12°C/m)
(Multiple Choice)
4.8/5  (39)
(39)
Explain the following, on the basis of osmosis or osmotic pressure: In trees and plants, water is drawn from the soil up into the branches and leaves.
(Essay)
4.8/5  (40)
(40)
What is the value for the van't Hoff factor for all nonelectrolytes?
(Multiple Choice)
4.9/5  (42)
(42)
Carbon tetrachloride, once widely used in fire extinguishers and as a dry cleaning fluid, has been found to cause liver damage to those exposed to its vapors over long periods of time. What is the boiling point of a solution prepared by dissolving 375 g of sulfur (S8, 256.5 g/mol) in 1250 g of CCl4? (For CCl4, Kb = 5.05°C/m; boiling point of pure CCl4 = 76.7°C)
(Multiple Choice)
4.9/5  (37)
(37)
Hydration is the process in which organic solvent molecules surround a solute particle.
(True/False)
4.9/5  (35)
(35)
When a nonvolatile solute is dissolved in a liquid, the vapor pressure exerted by the liquid decreases.
(True/False)
4.8/5  (41)
(41)
What mass of water is required to dissolve 25.31 g of potassium nitrate (KNO3) in order to prepare a 0.1982 m solution?
(Multiple Choice)
4.7/5  (33)
(33)
The solubility of nitrogen gas in water at 25°C and a nitrogen pressure of 522 mmHg is 4.7 × 10-4 mol/L. What is the value of the Henry's law constant in mol L-1• atm-1?
(Multiple Choice)
4.8/5  (31)
(31)
A solution is 40.00% by volume benzene (C6H6) in carbon tetrachloride at 20°C. The vapor pressure of pure benzene at this temperature is 74.61 mmHg and its density is 0.87865 g/cm3; the vapor pressure of pure carbon tetrachloride is 91.32 mmHg and its density is 1.5940 g/cm3. If this solution is ideal, its total vapor pressure at 20°C is
(Multiple Choice)
4.9/5  (39)
(39)
A solution of potassium hydroxide is in equilibrium with undissolved solute at 45°C. What will happen if the temperature is raised to 50°C? (ΔHsoln = -57.6 kJ/mol)
(Multiple Choice)
4.8/5  (29)
(29)
Which is true regarding the solvation of a solute in a solvent?
(Multiple Choice)
4.8/5  (41)
(41)
The solubility of oxygen in lakes high in the Rocky Mountains is affected by the altitude. If the aqueous solubility of atmospheric O2 is 2.67 × 10-4 M at sea level and 25°C, what is the solubility of O2 at an elevation of 12,000 ft where the total pressure is 0.657 atm? Assume the temperature is 25°C, and that the mole fraction of O2 in air is 0.209 at both 12,000 ft and at sea level.
(Multiple Choice)
4.8/5  (29)
(29)
Below is a diagram representing two chambers containing solvent A, separated by a semipermeable membrane (a dashed line) which allows only solvent A to pass through. 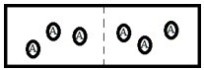 If a solute X is added to the chamber on the left, which diagram below best represents how the system will respond in order to reach a new equilibrium?
If a solute X is added to the chamber on the left, which diagram below best represents how the system will respond in order to reach a new equilibrium?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following aqueous solutions should demonstrate the most nonideal behavior?
(Multiple Choice)
4.9/5  (42)
(42)
What is the molarity of a solution prepared by diluting 1.85 L of 6.5 M KOH to 11.0 L?
(Multiple Choice)
4.8/5  (32)
(32)
Explain the following, on the basis of osmosis or osmotic pressure: Drinking salt water actually dehydrates our tissues.
(Essay)
4.8/5  (36)
(36)
Showing 121 - 140 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)