Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which combination of a 36.0% (w/w) stock solution of acetic acid (d = 1.045 g/mL) and water will result in 1.00 kg of a 15.0% (w/w) acetic acid solution?
(Multiple Choice)
4.8/5  (40)
(40)
Explain the following, on the basis of osmosis or osmotic pressure: Meat that is salted before cooking tends to dry out.
(Essay)
4.8/5  (35)
(35)
What is the molarity of a 17.0% by mass solution of sodium acetate, NaC2H3O2 (82.0 g/mol), in water? The density of the solution is 1.09 g/mL.
(Multiple Choice)
4.7/5  (37)
(37)
What volume of a 2.75 M solution of NaOH is required to make 500.0 mL of a 1.27 M solution of NaOH?
(Multiple Choice)
4.8/5  (36)
(36)
Aqueous ammonia is commercially available in a solution that is 28% (w/w) ammonia. What is the mole fraction of ammonia in such a solution?
(Multiple Choice)
4.9/5  (39)
(39)
A solution containing 0.102 g of an unknown compound dissolved in 100. mL of water has an osmotic pressure of 28.1 mmHg at 20.°C. What is the molar mass of the compound? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg)
(Multiple Choice)
4.7/5  (29)
(29)
Calcium nitrite is used as a corrosion inhibitor in lubricants. What is the molality of a solution prepared by dissolving 18.5 g of calcium nitrite in 83.5 g of distilled water?
(Multiple Choice)
4.8/5  (39)
(39)
Colligative properties are properties that depend on the number of solvent particles in solution, but not on the nature of the solvent.
(True/False)
4.7/5  (34)
(34)
What is defined as a solution that has a higher concentration of dissolved substances than plasma?
(Multiple Choice)
4.8/5  (42)
(42)
If the shell of a raw egg is carefully dissolved away, and the egg in its flexible membrane is then placed in distilled water, the egg's volume will expand. Explain.
(Essay)
4.9/5  (28)
(28)
Which substance is present in the smallest proportion in a solution?
(Multiple Choice)
4.9/5  (39)
(39)
The structure of Vitamin B1, thiamine, is shown here. Vitamin B1 is most soluble in ________. 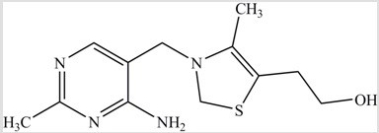
(Multiple Choice)
4.7/5  (44)
(44)
What is defined as the difference between the freezing point of a pure solvent and the freezing point of the solution?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 61 - 80 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)