Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Potassium fluoride is used for frosting glass. Calculate the molarity of a solution prepared by dissolving 78.6 g of KF in enough water to produce 225 mL of solution.
(Multiple Choice)
4.9/5  (44)
(44)
Osmosis is the selective passage of solvent molecules through a porous membrane from a more concentrated solution to a more dilute one.
(True/False)
5.0/5  (38)
(38)
Which response lists all the following pairs that are miscible liquids? I. octane (C8H18) and water
II) acetic acid (CH3COOH) and water
III) octane (C8H18) and carbon tetrachloride (CCl4)
(Multiple Choice)
4.7/5  (40)
(40)
What is the molality of a solution prepared by dissolving 84.7 g of KMnO4 in 165 g of water?
(Multiple Choice)
5.0/5  (34)
(34)
Hydrophilic and hydrophobic are not exactly the opposite phenomenon.
(True/False)
4.9/5  (41)
(41)
A(n) ________ ________ is the name given to the material which allows the passage of solvent molecules but blocks the passage of solute molecules.
(Short Answer)
4.9/5  (33)
(33)
At 10°C one volume of water dissolves 3.10 volumes of chlorine gas at 1.00 atm pressure. What is the Henry's law constant of Cl2 in water? (R = 0.0821 atm • L • mol-1• K-1 )
(Multiple Choice)
4.9/5  (45)
(45)
What mass of LiOH is required to prepare 0.250 L of a 3.55 M solution?
(Multiple Choice)
4.7/5  (35)
(35)
In the following diagrams, the black circles represent a solute in solution. Which diagram represents a saturated solution?
(Multiple Choice)
4.8/5  (38)
(38)
What relationship states that the partial pressure of a solvent over a solution is given by the vapor pressure of the pure solvent times the mole fraction of the solvent in the solution?
(Multiple Choice)
4.9/5  (32)
(32)
Below is a diagram representing a gas G in equilibrium, above a sample of water and dissolved in the water. 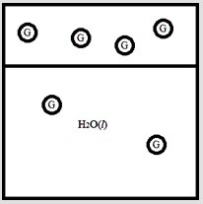 If the pressure of the gas G is doubled, which diagram below best represents how the system will respond?
If the pressure of the gas G is doubled, which diagram below best represents how the system will respond?
(Multiple Choice)
4.8/5  (40)
(40)
The solubility of gases in water ________ (increases or decreases) as the temperature increases.
(Short Answer)
4.8/5  (41)
(41)
What is the molarity of a solution of 10.0% by mass cadmium sulfate, CdSO4 (208.47 g/mol)? The density of the solution is 1.10 g/mL.
(Multiple Choice)
4.8/5  (41)
(41)
What mass of ethanol (C2H5OH), a nonelectrolyte, must be added to 10.0 L of water to give a solution that freezes at -10.0°C? Assume the density of water is 1.0 g/mL. (Kf of water is 1.86°C/m.)
(Multiple Choice)
5.0/5  (36)
(36)
The solubility of the oxidizing agent potassium permanganate is 7.1 g per 100.0 g of water at 25°C. What is the mole fraction of potassium permanganate (KMnO4) in this solution?
(Multiple Choice)
4.8/5  (34)
(34)
The mixing of solvent molecules and solute molecules is usually described as
(Multiple Choice)
4.9/5  (37)
(37)
Determine the freezing point of a solution which contains 0.31 mol of sucrose in 175 g of water. (For water, Kf = 1.86°C/m)
(Multiple Choice)
4.9/5  (33)
(33)
Which one of the following substances is the strongest electrolyte?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 41 - 60 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)