Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Variable melting point, variable hardness, and good conductor of heat and electricity are properties which describe a(n) ________ crystal.
(Short Answer)
4.9/5  (35)
(35)
In the packing of identical atoms with cubic unit cells, the packing efficiency increases as the coordination number increases.
(True/False)
4.8/5  (35)
(35)
Which one of the following substances crystallizes as a molecular solid?
(Multiple Choice)
4.9/5  (34)
(34)
Which substance will exhibit hydrogen bonding between molecules?
(Multiple Choice)
4.8/5  (45)
(45)
The atomic planes in a graphite crystal are separated by 335 pm. At what angle would you find the first-order (n = 1) diffraction of 0.154-nm X rays from a graphite crystal?
(Multiple Choice)
4.9/5  (31)
(31)
Give an example of an ionic crystal and a metallic crystal which contain an element common to each.
(Short Answer)
4.8/5  (37)
(37)
The location that indicates conditions under which two phases can exist in equilibrium is called the
(Multiple Choice)
4.7/5  (34)
(34)
________ ________ is a special type of dipole-dipole interaction.
(Short Answer)
4.8/5  (45)
(45)
The strongest intermolecular interactions between hydrogen fluoride (HF) molecules arise from
(Multiple Choice)
5.0/5  (39)
(39)
Use the graph of vapor pressure to determine the normal boiling point of O2. 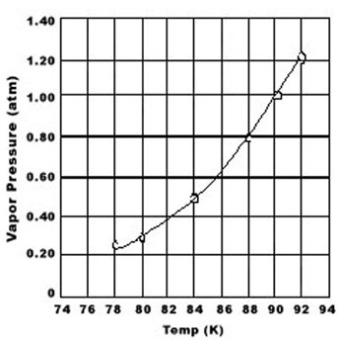
(Multiple Choice)
5.0/5  (42)
(42)
Iron crystallizes in the body-centered cubic lattice. What is the coordination number for Fe?
(Multiple Choice)
4.9/5  (29)
(29)
The energy of a hydrogen bond is greater than that of a typical covalent bond.
(True/False)
4.9/5  (33)
(33)
Identify the dominant (strongest) type of intermolecular force present in NH3(l).
(Short Answer)
4.7/5  (46)
(46)
Which of the following lacks a regular three-dimensional arrangement of atoms?
(Multiple Choice)
4.7/5  (34)
(34)
What is the energy in kJ/mol required to melt 1 mole of a solid?
(Multiple Choice)
4.9/5  (40)
(40)
Which substance has the highest vapor pressure at room temperature?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)