Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which one of the following pure substance has both dispersion forces and dipole-dipole forces?
(Multiple Choice)
4.7/5  (27)
(27)
The arrow in this phase diagram represents which phase change? 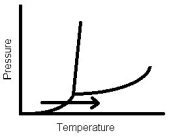
(Multiple Choice)
4.8/5  (41)
(41)
Which substance should exhibit hydrogen bonding in the liquid phase?
(Multiple Choice)
4.9/5  (28)
(28)
________ is the attraction of unlike molecules involved in capillary action.
(Short Answer)
4.7/5  (42)
(42)
Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm3 at 27°C. What is the atomic radius of Pd?
(Multiple Choice)
4.7/5  (38)
(38)
Below is the phase diagram for sulfur. Which phase has the highest density at 119°C? 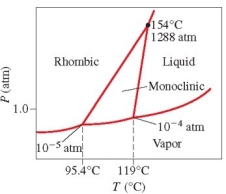
(Multiple Choice)
4.8/5  (38)
(38)
Ionic crystals are composed of charged spheres that are held together by covalent bonds.
(True/False)
4.8/5  (31)
(31)
Naphthalene sublimes at room temperature and 1 atmosphere pressure; which statement is true concerning the triple point of naphthalene?
(Multiple Choice)
4.9/5  (45)
(45)
The strongest intermolecular interactions between hydrogen sulfide (H2S) molecules arise from
(Multiple Choice)
4.9/5  (35)
(35)
If the adhesive forces between a liquid and the walls of a capillary tube are greater than the cohesive forces within the liquid,
(Multiple Choice)
4.9/5  (41)
(41)
A metal such as chromium in the body-centered cubic lattice has ________ atom(s) per unit cell.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the responses includes all of the following that can form hydrogen bonds with water molecules? I. Na+, II. CH3COOH, III. C2H6, IV. CH3NH2
(Multiple Choice)
4.8/5  (43)
(43)
Which kinds of intermolecular forces exist between propane molecules? 
(Multiple Choice)
4.9/5  (40)
(40)
What mass of water would need to evaporate from your skin in order to dissipate 1.70 ×105 J of heat from the surface of your body? H2O(l) → H2O(g) ΔHvap = 40.7 kJ/mol
(Multiple Choice)
4.8/5  (34)
(34)
Crystals of elemental sulfur are easily crushed, and melt at 113°C. Liquid sulfur does not conduct electricity. What kind of crystal is this?
(Short Answer)
4.9/5  (33)
(33)
The molar enthalpy of vaporization of boron tribromide is 30.5 kJ/mol, and its normal boiling point is 91°C. What is the vapor pressure of BBr3 at 20.°C? (R = 8.314 J/K • mol)
(Multiple Choice)
4.7/5  (38)
(38)
For which of the following pure substances are the intermolecular interactions entirely due to dispersion forces?
(Multiple Choice)
4.7/5  (35)
(35)
A face-centered crystal lattice has one atom in the center of the unit cell.
(True/False)
5.0/5  (42)
(42)
a. State the essential requirements for hydrogen bonding to be important in a compound.
b. List four properties of water which are significantly influenced by the presence of hydrogen bonding.
(Essay)
4.9/5  (40)
(40)
Showing 41 - 60 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)