Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Octane has a vapor pressure of 40. torr at 45.1°C and 400. torr at 104.0°C. What is its heat of vaporization? (R = 8.314 J/K • mol)
(Multiple Choice)
4.9/5  (39)
(39)
Identify the dominant (strongest) type of intermolecular force present in RbCl(s).
(Short Answer)
4.8/5  (31)
(31)
Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first.
(Multiple Choice)
4.7/5  (38)
(38)
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure. The edge length of this cubic unit cell is 540.9 pm. Determine the density of zincblende.
(Multiple Choice)
4.8/5  (31)
(31)
The surface tension of water is lowered when a detergent is present in solution.
(True/False)
4.8/5  (40)
(40)
In which of the following compounds will the molecules not form hydrogen bonds with each other?
(Multiple Choice)
4.7/5  (39)
(39)
a. Name the two unit cells which occur in close packing of identical atoms.
b. Briefly explain how the two types of close-packed lattices of identical atoms differ, in terms of atomic arrangements.
(Essay)
4.7/5  (20)
(20)
Which one of the following involves ion-dipole interactions?
(Multiple Choice)
4.8/5  (37)
(37)
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3. What is the radius of a platinum atom?
(Multiple Choice)
5.0/5  (39)
(39)
Choose the response that lists the member of each of the following pairs that has the higher boiling point. I. H2O or KI, II. HF or HI, III. Cl2 or Br2
(Multiple Choice)
4.8/5  (37)
(37)
Krypton has a higher melting point than argon because of its
(Multiple Choice)
4.8/5  (47)
(47)
Which one of the following substances does not exist in the indicated solid type?
(Multiple Choice)
4.7/5  (30)
(30)
At what temperature does ethanol boil on a day in the mountains when the barometric pressure is 547 mmHg? The heat of vaporization of ethanol is 39.3 kJ/mol and its normal boiling point is 78.3°C. (R = 8.314 J/K • mol)
(Multiple Choice)
4.9/5  (35)
(35)
The vapor pressure of ethanol is 400. mmHg at 63.5°C. Its molar heat of vaporization is 39.3 kJ/mol. What is the vapor pressure of ethanol, in mmHg, at 34.9°C? (R = 8.314 J/K • mol)
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following atoms does not participate in hydrogen bonding?
(Multiple Choice)
4.9/5  (40)
(40)
Poor conductor of heat and electricity, soft, and low melting point are properties which describe a(n) ________ crystal.
(Short Answer)
4.9/5  (32)
(32)
Based on the phase diagram of a pure substance given below, what phase exists at point F? 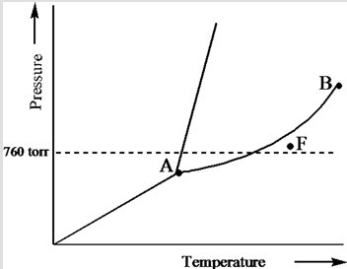
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following factors can contribute to the viscosity for a liquid?
(Multiple Choice)
4.9/5  (43)
(43)
Below is the phase diagram for sulfur. Which phase is present at 100°C and 1 atm? 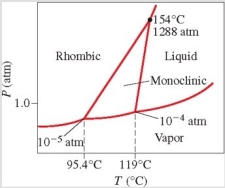
(Multiple Choice)
4.8/5  (44)
(44)
Showing 101 - 120 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)