Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
One of the crystalline forms of zinc oxide is a structure where the oxygen atoms are at the lattice points in a face-centered cubic lattice. How many atoms are in each unit cell?
(Multiple Choice)
4.9/5  (42)
(42)
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg. (R = 8.314 J/K • mol) 
(Multiple Choice)
4.8/5  (37)
(37)
What is the name given for the attraction between unlike molecules involved in capillary action?
(Multiple Choice)
4.7/5  (23)
(23)
Polyethylene plastic consists of long chains of carbon atoms, each of which is also bonded to hydrogens as shown below: 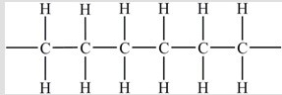 Water forms beads when placed on a polyethylene surface. Why?
Water forms beads when placed on a polyethylene surface. Why?
(Essay)
4.7/5  (43)
(43)
If liquid bromine is cooled to form a solid, which type of solid does it form?
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following is defined as the attractive forces between polar molecules? 
(Multiple Choice)
4.9/5  (39)
(39)
What name is given to a quantitative measure of the elastic force in the surface of a liquid?
(Multiple Choice)
4.8/5  (42)
(42)
Vanadium crystallizes in a body-centered cubic lattice, and the length of the edge of a unit cell is 305 pm. What is the density of V?
(Multiple Choice)
4.8/5  (47)
(47)
How much energy (heat) is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 
(Multiple Choice)
4.9/5  (25)
(25)
What is the intermolecular force that exists between a magnesium ion and hydrogen sulfide?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following pure substances has the strongest dispersion forces?
(Multiple Choice)
4.9/5  (42)
(42)
Octane, C8H18, boils at 125°C, whereas water boils at 100°C. This information suggests that the dispersion forces in nonpolar octane molecules are stronger than the dispersion forces and hydrogen bonding in water.
(True/False)
4.9/5  (27)
(27)
________ ________ are the attractions that hold particles together in condensed phases.
(Short Answer)
4.9/5  (38)
(38)
If a molecule at the surface of a liquid has enough kinetic energy to escape the liquid phase and enter the gas phase, then which of the following terms is used to describe this phenomenon?
(Multiple Choice)
4.8/5  (35)
(35)
Below is a representation of liquid water in equilibrium with its water vapor in a rigid container at 35ºC. The circles represent water vapor. 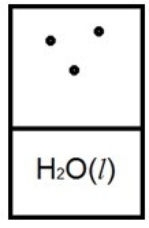 Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol. (R = 8.314 J/K • mol)
Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol. (R = 8.314 J/K • mol)
(Multiple Choice)
4.9/5  (41)
(41)
The triple point of iodine is at 0.12 atm and 115°C. Thus, liquid I2
(Multiple Choice)
4.9/5  (39)
(39)
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
(Multiple Choice)
4.8/5  (34)
(34)
What is the attractive force between like molecules involved in capillary action?
(Multiple Choice)
4.9/5  (39)
(39)
Which would be expected to have the highest surface tension at room temperature?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 81 - 100 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)