Exam 6: The Behavior of Proteins Enzymes
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
What effect is seen on a Lineweaver-Burk graph when a competitive inhibitor is added?
(Multiple Choice)
4.8/5  (44)
(44)
The initial rate of an enzymatic reaction is usually determined in order to assure that
(Multiple Choice)
4.9/5  (35)
(35)
If an inhibitor changes the slope of the Lineweaver-Burk graph, but not the x-intercept, it is this type of inhibition:
(Multiple Choice)
4.8/5  (40)
(40)
The order of a reaction can be determined from the balanced equation for the reaction.
(True/False)
4.7/5  (34)
(34)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 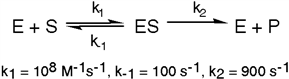 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the Vmax of the enzyme?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the Vmax of the enzyme?
(Multiple Choice)
4.8/5  (31)
(31)
In the reaction catalyzed by aspartate transcarbamoylase, a graph in which the rate is plotted against the concentration of substrate
(Multiple Choice)
4.8/5  (32)
(32)
What effect is seen on a Lineweaver-Burk graph when a non-competitive inhibitor is added?
(Multiple Choice)
4.8/5  (42)
(42)
If the y-intercept of a Lineweaver-Burk plot = 1.91 (sec\millimole) and the slope = 75.3 L\sec, KM equals:
(Multiple Choice)
4.8/5  (34)
(34)
Given the rate law, rate = k[A][B], the overall reaction order is
(Multiple Choice)
4.8/5  (34)
(34)
If an inhibitor changes the slope of the Lineweaver-Burk graph, but not the y-intercept, it is this type of inhibition:
(Multiple Choice)
4.9/5  (32)
(32)
What effect does a catalyst have on the Δ G ° of a reaction?
(Multiple Choice)
4.9/5  (40)
(40)
What effect is seen on a Lineweaver-Burk graph when a mixed-type inhibitor is added?
(Multiple Choice)
4.7/5  (41)
(41)
Showing 61 - 80 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)