Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
Which of the following coenzymes is not a carrier of electrons in biological redox reactions?
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
A
The body allows energy consuming reactions to occur by coupling them with reactions which have a negative Δ G.
Free
(True/False)
4.8/5  (34)
(34)
Correct Answer:
True
The production of larger molecules from smaller ones is called
(Multiple Choice)
4.8/5  (43)
(43)
In order to initiate many metabolic pathways it is necessary to activate the starting materials.
(True/False)
4.9/5  (31)
(31)
The energy released during metabolism of nutrients can be used to synthesize ATP from ADP and phosphate.
(True/False)
4.8/5  (38)
(38)
Exhibit 15A Consider the reaction of alcohol dehydrogenase. 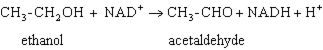 Refer to Exhibit 15A. Which molecule loses electrons?
Refer to Exhibit 15A. Which molecule loses electrons?
(Multiple Choice)
4.8/5  (31)
(31)
Among the following organophosphates, which compound has the highest amount of free energy of hydrolysis?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following is not a mechanism used to activate substrates for further metabolism?
(Multiple Choice)
4.9/5  (30)
(30)
Biochemists use a modified value for standard Δ G values because
(Multiple Choice)
4.8/5  (33)
(33)
When we say that the efficiency of glycolysis is about 33% we mean that
(Multiple Choice)
4.9/5  (35)
(35)
I am performing a reaction, A → B, with Δ G ° ' = − 5000 kJ\mol. I start the reaction with 10 mM A and no B. After allowing the reaction to proceed for 24 hrs at room temperature and atmospheric pressure, I analyze a sample of the reaction mix to find I now have 8 mM A and 2 mM B. Which of the following conclusions should I make?
(Multiple Choice)
5.0/5  (44)
(44)
The efficiency of aerobic metabolism is greater than that of anaerobic metabolism even though much more energy is released in aerobic than in anaerobic metabolism because
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)