Exam 2: Water the Solvent for Biochemical Reactions
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
Exhibit 2A The structure of ATP with various groups labeled. Group III is the entire phosphate group. 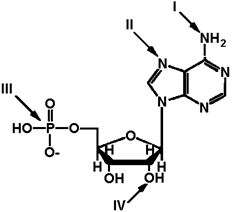 Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
Free
(Multiple Choice)
4.7/5  (46)
(46)
Correct Answer:
E
What is the pH of a solution with [H+] = 10 mM?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
C
Exhibit 2B Contains information on the pK's of some common buffers. 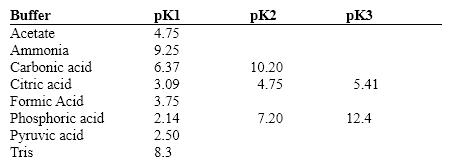 Which of the following is important to know when deciding if a given buffer will be effective for an experiment?
Which of the following is important to know when deciding if a given buffer will be effective for an experiment?
(Multiple Choice)
4.9/5  (23)
(23)
Distinguish between the hydrogen bonding found in liquid water and ice.
(Essay)
4.8/5  (31)
(31)
Which of the following acids would serve as a good buffer for a reaction at pH = 8.0? 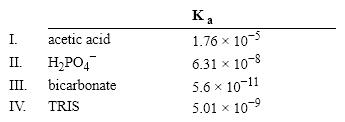
(Multiple Choice)
4.9/5  (37)
(37)
Exhibit 2B Contains information on the pK's of some common buffers. 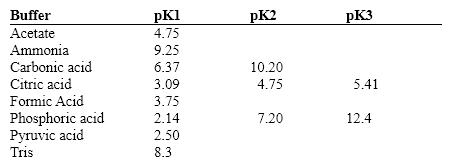 Refer to Exhibit 2B. An ammonium buffer would work well at this pH:
Refer to Exhibit 2B. An ammonium buffer would work well at this pH:
(Multiple Choice)
4.9/5  (32)
(32)
True hydrogen bonds can NOT form between hydrogen and this element:
(Multiple Choice)
4.9/5  (37)
(37)
The non-covalent interaction below associated with the strongest force in aqueous solution is
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following equations represents the relationship between the p Ka of an acid and the pH of a solution containing the acid and its conjugate base?
(Multiple Choice)
4.7/5  (34)
(34)
Nonphysiological buffers such as HEPES and PIPES have come into common use because
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is not considered a van der Waal's force?
(Multiple Choice)
4.9/5  (47)
(47)
Buffers which lack biological activity and are unlikely to interfere with any biochemical reactions include:
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)