Exam 6: The Behavior of Proteins Enzymes
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
The main difference between a catalyzed and an uncatalyzed reaction is that
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 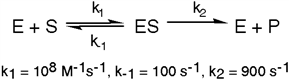 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
In the following graph, which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
In the following graph, which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate? 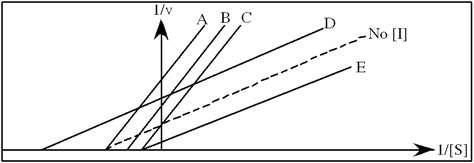
Free
(Multiple Choice)
4.7/5  (41)
(41)
Correct Answer:
A
Which of the following diseases has not been successfully treated using the principles of enzyme inhibition?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
Which of the following is true about the enzyme chymotrypsin?
(Multiple Choice)
4.9/5  (44)
(44)
To study the nature of an enzyme, Vmax is not as good a measurement as the catalytic rate constant kcat because:
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is true about a mixed type inhibition?
(Multiple Choice)
4.9/5  (34)
(34)
The E-S complex often shows as a slight depression in the energy profile for the reaction.
(True/False)
4.9/5  (33)
(33)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 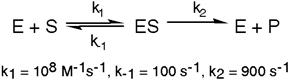 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the uncatalyzed reaction?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the uncatalyzed reaction?
(Multiple Choice)
4.8/5  (34)
(34)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 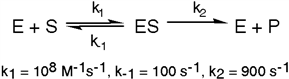 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
(Multiple Choice)
4.8/5  (29)
(29)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 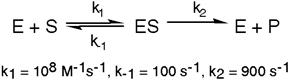 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
(Multiple Choice)
5.0/5  (47)
(47)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 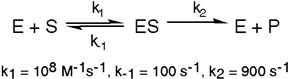 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the actual velocity of the forward reaction under physiologic conditions?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the actual velocity of the forward reaction under physiologic conditions?
(Multiple Choice)
4.8/5  (36)
(36)
The amount of energy released during a reaction tells nothing about the rate at which that reaction will occur.
(True/False)
4.7/5  (31)
(31)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)