Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism
Exam 1: Biochemistry and the Organization of Cells76 Questions
Exam 2: Water the Solvent for Biochemical Reactions90 Questions
Exam 3: Amino Acids and Peptides80 Questions
Exam 4: The Three Dimensional Structure of Proteins87 Questions
Exam 5: Protein Purification and Characterization Techniques72 Questions
Exam 6: The Behavior of Proteins Enzymes88 Questions
Exam 7: The Behavior of Proteins Enzymes Mechanisms and Control86 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes95 Questions
Exam 9: Nucleic Acids How Structure Conveys Information71 Questions
Exam 10: Biosynthesis of Nucleic Acids Replication91 Questions
Exam 11: Transcription of the Genetic Code the Biosynthesis of Rna103 Questions
Exam 12: Protein Synthesis Translation of the Genetic Message90 Questions
Exam 13: Nucleic Acid Biotechnology Techniques99 Questions
Exam 14: Viruses Cancer and Immunology47 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism65 Questions
Exam 16: Carbohydrates97 Questions
Exam 17: Glycolysis72 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism87 Questions
Exam 19: The Citric Acid Cycle85 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation71 Questions
Exam 21: Lipid Metabolism100 Questions
Exam 22: Photosynthesis79 Questions
Exam 23: The Metabolism of Nitrogen83 Questions
Exam 24: Integration of Metabolism Cellular Signaling73 Questions
Select questions type
Exhibit 15A Consider the reaction of alcohol dehydrogenase. 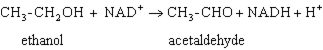 Refer to Exhibit 15A. Which molecule is reduced?
Refer to Exhibit 15A. Which molecule is reduced?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following are examples of endergonic processes?
(Multiple Choice)
4.7/5  (31)
(31)
Many cells oxidize fatty acids to produce ATP. If no ATP were produced, the Δ G ° ' of this process would be
(Multiple Choice)
4.8/5  (35)
(35)
If the reaction A → B has Δ G = +25 Joule\mol and the reaction B → C has Δ G = − 15 Joule\mol, the overall energy change A → C will be
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following is not true concerning standard states?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following are examples of exergonic processes?
(Multiple Choice)
4.9/5  (29)
(29)
A biological reaction with a significantly postive Δ G under cellular conditions may proceed in the forward direction because
(Multiple Choice)
4.9/5  (35)
(35)
What happens to the entropy of a molecule as the number of resonance structures increases?
(Multiple Choice)
4.9/5  (42)
(42)
Which best describes the Δ G for hydrolysis of creatine phosphate under cellular conditions in which the concentration of creatine phosphate, creatine, and phosphate all equal 1 mM at 25 ° C. The Δ G ° for the hydrolysis of creatine phosphate at 25 ° C is − 43 kJ\mol.
(Multiple Choice)
4.7/5  (33)
(33)
The phosphorylation of ADP to produce ATP is endergonic because
(Multiple Choice)
4.8/5  (38)
(38)
In order to drive the synthesis of ATP, the hydrolysis of an organic phosphate
(Multiple Choice)
4.8/5  (38)
(38)
Consider this rxn which has a Δ G ° = +0.4 kJ\mol.
A + B ↔ C + D
1 M A, 1 M B, 0.1 M C and 0.1 M D are added to a container at room temperature. Which of the following statements is true?
(Multiple Choice)
4.8/5  (36)
(36)
The standard state usually used in biochemistry ( Δ G ° ') includes
(Multiple Choice)
4.8/5  (28)
(28)
Showing 21 - 40 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)