Exam 2: The Chemistry of Living Things
Exam 1: Human Biology, Science, and Society69 Questions
Exam 2: The Chemistry of Living Things100 Questions
Exam 3: Structure and Function of Cells107 Questions
Exam 4: From Cells to Organ Systems88 Questions
Exam 5: The Skeletal System74 Questions
Exam 6: The Muscular System83 Questions
Exam 7: Blood95 Questions
Exam 8: Heart and Blood Vessels107 Questions
Exam 9: The Immune System and Mechanisms of Defense102 Questions
Exam 10: The Respiratory System: Exchange of Gases81 Questions
Exam 11: The Nervous System: Integration and Control100 Questions
Exam 12: Sensory Mechanisms83 Questions
Exam 13: The Endocrine System82 Questions
Exam 14: The Digestive System and Nutrition80 Questions
Exam 15: The Urinary System70 Questions
Exam 16: Reproductive Systems89 Questions
Exam 17: Cell Reproduction and Differentiation78 Questions
Exam 18: Cancer: Uncontrolled Cell Division and Differentiation77 Questions
Exam 19: Genetics and Inheritance71 Questions
Exam 20: DNA Technology and Genetic Engineering71 Questions
Exam 21: Development and Aging72 Questions
Exam 22: Evolution and the Origins of Life78 Questions
Exam 23: Ecosystems and Populations74 Questions
Exam 24: Human Impacts, Biodiversity, and Environmental Issues71 Questions
Select questions type
Which one of the following statements is TRUE regarding the structure of the atom?
Free
(Multiple Choice)
4.7/5  (28)
(28)
Correct Answer:
D
All things on Earth are made up of ________, which is defined as anything that has mass and occupies space.
Free
(Short Answer)
4.8/5  (35)
(35)
Correct Answer:
matter
Chlorine has an atomic number of 17 and an atomic mass of 35. Therefore, chlorine has ________ electrons and ________ neutrons.
Free
(Multiple Choice)
4.9/5  (25)
(25)
Correct Answer:
A
A substance that helps to maintain a stable pH is a(n) ________.
(Short Answer)
4.9/5  (32)
(32)
Each of the following statements about carbon is TRUE EXCEPT which one?
(Multiple Choice)
4.8/5  (35)
(35)
In order for a cell to produce a fat, it must have one molecule of ________ and three ________.
(Short Answer)
4.8/5  (32)
(32)
Large organic molecules that are composed of thousands of smaller molecules bonded to one another are known as ________.
(Short Answer)
4.8/5  (23)
(23)
Which one of the following occurs when a phosphate is removed from an ATP molecule?
(Multiple Choice)
4.9/5  (29)
(29)
Which one of the following statements accurately describes hydrolysis reactions in biological systems?
(Multiple Choice)
4.9/5  (33)
(33)
________ bonds hold the hydrogens to the oxygen within a water molecule, and ________ bonds attract one water molecule to other water molecules.
(Multiple Choice)
5.0/5  (41)
(41)
Use the letters from the figure below to answer the following questions.
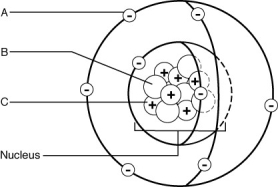 -Isotopes of this element would differ in the number of ________.
-Isotopes of this element would differ in the number of ________.
(Short Answer)
4.9/5  (34)
(34)
The universal energy source for cells is a nucleotide known as ________.
(Short Answer)
5.0/5  (42)
(42)
The normal pH of human blood falls within a range that is near a(n) ________ pH.
(Short Answer)
4.9/5  (36)
(36)
If the number of protons in an atom equals the number of electrons in the atom, the atom is an ion.
(True/False)
4.7/5  (42)
(42)
Each amino acid is composed of a central carbon that forms covalent bonds with four other atoms/molecules. These atoms/molecules include each of the following EXCEPT which one?
(Multiple Choice)
4.7/5  (35)
(35)
A mad scientist has ripped apart an atom and collected all the subatomic particles located in the nucleus of the atom. Which one of the following has he collected?
(Multiple Choice)
4.9/5  (37)
(37)
Which one of the following statements CORRECTLY describes the relationship between an atom and an element?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)