Exam 2: Atoms and Elements
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
The element that is essential for human nutrition and is responsible for formation of bones and teeth is ___.
(Short Answer)
4.9/5  (33)
(33)
Gamma rays require a thick slab of concrete or lead to block them because they
(Multiple Choice)
4.9/5  (33)
(33)
Define the term element. Give one example of an element that is a metal, an element that is a nonmetal, and an element that is a semimetal.
(Short Answer)
4.7/5  (36)
(36)
If one starts with 10.0 grams of a radioactive substance, how much will remain after 3 half lives?
(Multiple Choice)
4.8/5  (35)
(35)
The number of moles of calcium (Ca) represented by 7.39 g of calcium is
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following statements is true about a beta particle?
(Multiple Choice)
4.7/5  (31)
(31)
Trace elements and vitamins are required for the body to function properly
(True/False)
4.8/5  (36)
(36)
A Nutrition Facts label on a food package indicates that one serving contains 2% of the recommended daily allowance (RDA) of iodine. If the serving size is one cup and the RDA for iodine is 0.15 mg per day, calculate the amount (in milligrams) supplied by one serving.
(Multiple Choice)
4.8/5  (29)
(29)
In an alpha therapy, Radium-223is used as a radioactive -emitter. The correct nuclear equation for this emission event is?
(Multiple Choice)
4.9/5  (35)
(35)
The number of ___ is the same in one mole of sodium as in one mole of zinc.
(Short Answer)
4.9/5  (30)
(30)
A patient who has undergone a PET scan that used fluorine-18 tagged glucose is likely to emit which of the following a few hours after the procedure?
(Multiple Choice)
4.9/5  (30)
(30)
Describe two ways to protect oneself from exposure to radiation when working in an environment that contains radioactive sources.
(Essay)
4.9/5  (34)
(34)
Iron-59 has a half-life of 45 days. How much of a 50.0-gram sample would remain after 180 days?
(Multiple Choice)
4.8/5  (26)
(26)
The higher up within a group or family on the periodic table,
(Multiple Choice)
4.8/5  (35)
(35)
If radium, 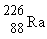 , were to emit an alpha particle, what would be the other product?
, were to emit an alpha particle, what would be the other product?
(Multiple Choice)
4.7/5  (40)
(40)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)