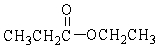Exam 5: Reactions
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
An oxidizing agent is the reactant in an oxidation - reduction reaction that causes reduction of another reactant by providing electrons for the other reactant to accept.
Free
(True/False)
4.8/5  (40)
(40)
Correct Answer:
False
A chemical reaction in which there is a transfer of electrons from one reactant to another reactant can be classified as
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
Methanol burns in air according to the equation below. How many grams of methanol can be burned by 10 grams of oxygen? 
(Multiple Choice)
4.9/5  (33)
(33)
In the following reaction, how many grams of water are needed to completely hydrolyze 30.8 g of the ester? 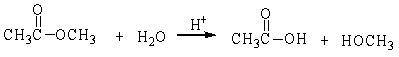
(Multiple Choice)
4.9/5  (35)
(35)
If there is a "limiting reactant" in the hypothetical reaction,
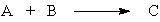 it is the substance
it is the substance
(Multiple Choice)
4.8/5  (37)
(37)
Benzoyl peroxide can be used as a treatment for acne. Benzoyl peroxide
(Multiple Choice)
4.9/5  (37)
(37)
If 5.5 moles of pentane are burned according to the following equation:
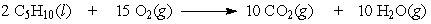 how many moles of oxygen are required?
how many moles of oxygen are required?
(Multiple Choice)
4.9/5  (41)
(41)
According to the following equation, what mass of silver nitrate would be required to react with 0.500 grams potassium chloride? 
(Multiple Choice)
4.8/5  (30)
(30)
2-methylcyclopentene reacts with hydrogen in the presence of platinum catalyst to form methylcyclopentane.
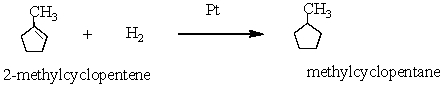 In this reaction:
In this reaction:
(Multiple Choice)
4.8/5  (32)
(32)
While this isn't a real reaction, we can say that carbonic acid, H2CO3, can be produced directly from the elements. Choose the statement that is correct when the given masses are supplied to react.
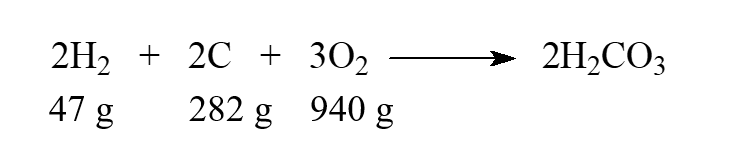
(Multiple Choice)
4.8/5  (30)
(30)
Balance the equation. Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
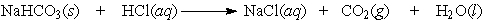
(Essay)
4.9/5  (41)
(41)
Write and balance the equation for the complete combustion of pentane (C5H12).
(Essay)
4.7/5  (37)
(37)
An oxidation reaction can be defined as when an atom loses an oxygen.
(True/False)
4.7/5  (27)
(27)
Sodium stearate is a soap that is produced from stearic acid, one of the fatty acids, by the reaction:
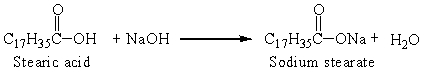 The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
(Multiple Choice)
4.9/5  (31)
(31)
Aluminum carbonate was once the active ingredient in Rolaids . It reacts with stomach acid according to the following equation:
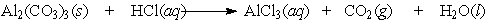 A single aluminum carbonate tablet weighs 2.48 g. How many grams of AlCl3 would be produced by the reaction of a single tablet?
A single aluminum carbonate tablet weighs 2.48 g. How many grams of AlCl3 would be produced by the reaction of a single tablet?
(Short Answer)
4.8/5  (47)
(47)
A test used by geologists to determine if the carbonate ion is present in rock can be performed by dripping a hydrochloric acid solution on the sample and looking for the bubbling of carbon dioxide. How many moles of carbon dioxide are produced by the reaction of 0.75 moles of limestone, CaCO3? 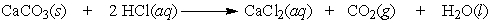
(Multiple Choice)
4.9/5  (32)
(32)
Tums tablets are composed of the substance calcium carbonate. Two tablets have a combined mass of 5.0 grams. Calcium carbonate reacts with and neutralizes excess stomach acid (hydrochloric acid). The products of this reaction are: water, calcium chloride, and carbon dioxide. Calculate the mass in grams of the hydrochloric acid that can be neutralized by 5.0 grams of calcium carbonate.
(Short Answer)
4.9/5  (33)
(33)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)