Exam 16: Acids and Bases, a Molecular Look
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Which of the following would form a basic solution when added to water?
(Multiple Choice)
4.7/5  (33)
(33)
For the reaction, CO2 + H2O  H2CO3, which of the following statements is incorrect?
H2CO3, which of the following statements is incorrect?
(Multiple Choice)
4.8/5  (45)
(45)
What is the formula for the conjugate base of the HCO3-(aq)ion?
(Short Answer)
4.7/5  (32)
(32)
Five solutions were prepared by dissolving 0.00100 mole of each of the following in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
(Multiple Choice)
4.8/5  (44)
(44)
What is the difference between a xerogel and aerogel solid made using the sol-gel process?
(Short Answer)
4.9/5  (36)
(36)
Explain how NH3 can be both a Brønsted-Lowry base and a Lewis base, at the same time.
(Essay)
4.9/5  (31)
(31)
For the system HC6H5O + C4H7O2-  C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following would form an acidic solution when added to water?
(Multiple Choice)
4.7/5  (37)
(37)
For the system NH2OH(aq)+ CH3NH3+(aq)  CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
(Short Answer)
4.9/5  (39)
(39)
Five solutions were prepared by dissolving 0.00100 mole of each of the following compounds in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
(Multiple Choice)
4.9/5  (36)
(36)
Selenium atoms can react with the sulfite ion to produce a selenosulfate ion, as shown below. 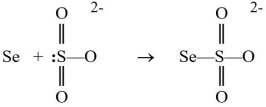 Which reactant is functioning as the Lewis base?
Which reactant is functioning as the Lewis base?
(Short Answer)
4.9/5  (25)
(25)
Given the following substances, listed in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the weakest base?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 21 - 40 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)