Exam 16: Acids and Bases, a Molecular Look
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
For the system H3PO4(aq)+ COOH-(aq)  HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
(Short Answer)
4.8/5  (28)
(28)
The oxide ion can react with the carbon dioxide molecule to produce a carbonate ion, as shown be low. 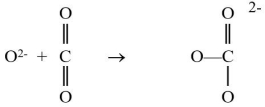 Which reactant is functioning as the Lewis acid?
Which reactant is functioning as the Lewis acid?
(Short Answer)
4.8/5  (38)
(38)
What is the formula for the conjugate acid of the HCO3-(aq)ion?
(Short Answer)
4.7/5  (41)
(41)
Which pair of reactants will yield H2SO4 as one of its products?
(Multiple Choice)
4.8/5  (48)
(48)
Water can be purified by many ways, including by distillation. A student distills water in a lab experiment, in which she is going to work with pH and acid/base properties. She obtains a pH meter, and discovers that her distilled water sample has an acidic pH reading. Knowing that neutral water should be neither acidic nor basic, the student assumes that the pH meter is not calibrated, but after calibrating it again, it reads a similar pH. What could explain the student's observations?
(Essay)
4.8/5  (39)
(39)
In the reaction, HSO4- + CN-  HCN + SO42-, which two species are acids?
HCN + SO42-, which two species are acids?
(Multiple Choice)
5.0/5  (40)
(40)
In the reaction, HClO3 + N2H4  ClO3- + N2H5+, which species are a conjugate acid-base pair?
ClO3- + N2H5+, which species are a conjugate acid-base pair?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the two species, H2S or HBr, is the stronger Brønsted-Lowry acid?
(Short Answer)
4.8/5  (37)
(37)
Given that X is the same atom for each of the following, which is the weakest oxoacid?
(Multiple Choice)
5.0/5  (39)
(39)
The step in the sol-gel process that forms oxygen bridges while forming water molecules can be best classified as
(Multiple Choice)
5.0/5  (42)
(42)
Would a solution of Al(NO3)3 be expected to form an acidic or basic solution in water?
(Short Answer)
4.7/5  (34)
(34)
In the reaction, HClO3(aq)+ N2H4(aq)  ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
(Short Answer)
4.8/5  (33)
(33)
Given that X is the same atom for each of the following, which is the strongest oxyacid?
(Multiple Choice)
4.8/5  (36)
(36)
In order to make a ceramic material with uniform composition and high structural integrity, the ________ process is preferred over the traditional heating process, which causes sintering.
(Short Answer)
4.8/5  (39)
(39)
Explain why the anion HCO3- can be classified as an amphoteric species.
(Essay)
4.8/5  (35)
(35)
Which of the following would form a basic solution when added to water?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 41 - 60 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)