Exam 16: Acids and Bases, a Molecular Look
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
What effect would bubbling SO3 gas through a solution of distilled water have on the pH of a solution?
(Short Answer)
4.9/5  (36)
(36)
The sulfide ion can react with the sulfur trioxide molecule to produce a thiosulfate ion, as shown below. 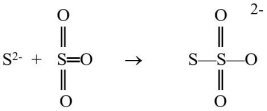 Which reactant is functioning as the Lewis base?
Which reactant is functioning as the Lewis base?
(Short Answer)
4.9/5  (39)
(39)
For the system HC6H5O + C4H7O2-  C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?
(Multiple Choice)
4.8/5  (35)
(35)
For the system H3PO4(aq)+ COOH-(aq)  HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
(Short Answer)
4.8/5  (46)
(46)
The strongest acid that can exist in aqueous solution is ________.
(Short Answer)
4.8/5  (39)
(39)
In the reaction, HSO4- + HS-  H2S + SO42-, which two species are acids?
H2S + SO42-, which two species are acids?
(Multiple Choice)
4.8/5  (42)
(42)
In the reaction, HSO4- + HS-  H2S + SO42-, which two species are bases?
H2S + SO42-, which two species are bases?
(Multiple Choice)
4.8/5  (34)
(34)
Given the following substances, listed in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the strongest base?
(Multiple Choice)
4.8/5  (36)
(36)
Given that X is the same atom for each of the following, which is the strongest oxyacid?
(Multiple Choice)
4.9/5  (49)
(49)
Showing 61 - 80 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)