Exam 11: Properties of Gases
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
What is the total pressure exerted by a gaseous mixture that consists of 8.00 g of methane and 8.00 g of ethane (C2H6)in a 3.50-liter container maintained at 35.20 °C?
(Multiple Choice)
5.0/5  (38)
(38)
A sample of a gas in a cylindrical chamber with a movable piston occupied a volume of 4.626 liters when the pressure was 0.983 atm and the temperature was 27.2°C. The pressure was readjusted to 1.388 atm by moving the piston. What is the volume occupied by the sample under the new conditions if the temperature remained constant throughout?
(Multiple Choice)
4.9/5  (45)
(45)
8.25 g of liquid hexane (C6H14)is introduced into a 10.0 L vessel containing 12.75 atm of oxygen gas at 25°C and ignited, yielding carbon dioxide and water. If the vessel is then cooled to −10°C, what will be the gas pressure inside the vessel?Hint: Consider the product states at the final temperature.
(Short Answer)
4.9/5  (40)
(40)
A gas sample weighing 8.280 grams occupies a volume of 4.260 liters at STP. What is the molecular mass of the sample?
(Short Answer)
4.9/5  (38)
(38)
A special shipping container for compressed gas is rated at 18.50 × 103 kPa. What is this rating in kbar?(1 atm = 101325 Pa = 760 torr = 760 mm Hg = 1.01325 bar = 1013.25 mb)
(Multiple Choice)
4.8/5  (41)
(41)
N2O is the gas found in aerosol cans of whipped cream. What is a similarity between N2O and CO2 that could make CO2 suitable for this use and why is N2O used anyway instead of CO2?
(Short Answer)
4.9/5  (43)
(43)
A pressure that will support a column of Hg to a height of 263 mm would support a column of water to what height? The density of mercury is 13.6 g/cm3; the density of water is 1.00 g/cm3.
(Short Answer)
4.8/5  (41)
(41)
A Torricelli barometer containing mercury is placed in a chamber used for training astronauts. The chamber is maintained at an air pressure of 1.20 × 104 Pa. What height should the Torricelli barometer in the chamber register?
(Short Answer)
4.7/5  (34)
(34)
A gaseous element has a density of 1.098 g L−1 when the temperature is 31.4 °C and the pressure is 744.5 torr. Which element best fits the description?
(Short Answer)
4.9/5  (40)
(40)
If container "A"is occupied by 1.00 mole of oxygen gas while container "B"is occupied by 20.0 grams of nitrogen gas and both containers are maintained at 0.00 °C and 650 torr then,
(Multiple Choice)
4.8/5  (31)
(31)
A closed-end manometer that utilizes an oil having a density of 0.820 g ml−1 is being used to measure gas pressure in a vessel designed for studies on the gaseous state. In a particular experiment, the oil in the closed end of this manometer was 62.2 cm above the U-neck, while the level in the end connected to the vessel was measured as 25.2 cm above the U-neck. What is the pressure, in atmospheres, of the gas?Hint: The density of mercury is 13.533 g mL−1 at room temperature.
(Short Answer)
4.8/5  (36)
(36)
A gas container has a volume of 5.604 L. It contains carbon monoxide gas at 28.2 °C. The pressure in the container is 868.5 torr. If the gas behaves as an ideal gas, how much should the gas sample weigh?
(Multiple Choice)
4.8/5  (42)
(42)
What is the gas pressure of the gas sample hooked up to the mercury manometer shown below if the atmospheric pressure is 749 mmHg? 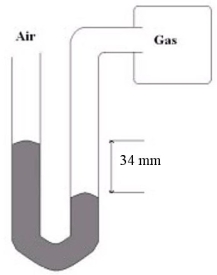
(Short Answer)
4.9/5  (42)
(42)
A gas mixture contains 5.00 liters of a mixture of nitrogen gas and oxygen gas. The total pressure in the tank is 38.6 atm when the temperature is 25.0 °C, and the mole fraction of oxygen is 0.400. How much does the oxygen in the tank weigh?
(Multiple Choice)
4.9/5  (40)
(40)
Water vapor is a polar gas molecule. Because of this, it has fairly strong interactions with itself in the gas phase that can make it behave less like an ideal gas that other gases under similar conditions. What is the difference between the calculated pressures found using the ideal gas law and those found using the van der Waals gas equation for 1 mole of water vapor at −100°C in a 1.00 L container?(van der Waals constants for water are a = 5.464 L2 atm mol−2, b = 0.03049 L mol−1)
(Short Answer)
4.8/5  (34)
(34)
Individuals are often advised to put out flames if they "smell gas."In reality, people smell dimethyl sulfide ((CH3)2S)that is added to natural gas to give it a detectable odor; natural gas by itself is odorless. What chemical characteristic of methyl sulfide makes it better to use as a gas indicator compared with the more pleasant-smelling isoamyl acetate (C7H14O2), also known as oil of banana?
(Short Answer)
4.8/5  (35)
(35)
A sample of a gas in a cylindrical chamber with a movable piston occupied a volume of 8.50 liters when the pressure was 9.85 × 104 Pa and the temperature was 24.9°C. The volume of the system was readjusted to 11.6 liters by moving the piston. What is the pressure exerted on the surface of the piston by the gas if the temperature of the system remained constant?
(Multiple Choice)
4.8/5  (32)
(32)
Equal masses of oxygen gas and HBr gas were placed in separate 1 L containers. If the gas samples are at the same temperature the oxygen sample will have a pressure that is ________ than the pressure of the HBr sample.
(Short Answer)
4.9/5  (43)
(43)
Calculate the volume of hydrogen gas at 0°C and 2.00 atm that will be formed when 245 mL of 0.735 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
(Short Answer)
4.8/5  (36)
(36)
A chemical reaction, A(s) B(s)+ C(g), occurs when substance A is strongly heated. The molecular mass of the gaseous product was to be determined from the following experimental data: Mass of A before reaction starts: 4.962 g
Mass of A after reaction finishes: 0 g Mass of residue (B)after cooling and weighing when no more gas was evolved: 3.684 g When all of the gas C evolved was collected and stored in a 658.5 mL glass vessel at 30.4 °C, the gas exerted a pressure of 748.5 torr.From this data, determine the apparent molecular mass of C, assuming it behaves as an ideal gas.
(Multiple Choice)
4.9/5  (43)
(43)
Showing 141 - 160 of 162
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)