Exam 11: Properties of Gases
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
A gaseous substance effuses twice as rapidly as sulfur dioxide gas. The gas could be
(Multiple Choice)
4.8/5  (37)
(37)
An open-end mercury manometer was constructed from a U-shaped tube and connected to a gas container. In a particular measurement, the level in the end of the tube connected to the gas container measured 82.8 cm above the U-neck, while the level in the open end (to the atmosphere)was 17.2 cm above the U-neck. The outside air pressure in the laboratory was measured as 764 torr. What is the pressure in the gas container?
(Multiple Choice)
4.9/5  (43)
(43)
For a substance that remains a gas under all the conditions listed, deviations from the expected values found using the ideal gas law would be the greatest at
(Multiple Choice)
4.8/5  (40)
(40)
The density of a gas sample was found to be 3.51 g/L. If the volume of the sample was changed from 1.00 L to 0.20 L under constant temperature, what would be the new density of the sample?
(Short Answer)
5.0/5  (43)
(43)
In order for a gas to be truly considered as "ideal,"the molecules of the gas ________ interact with each other and ________ volume.
(Short Answer)
4.8/5  (35)
(35)
Mountain climbers often need to take tanks of oxygen with them as they approach higher altitudes. Explain why this oxygen is needed by the climbers.
(Essay)
5.0/5  (38)
(38)
The van der Waals equation of state for a real gas is 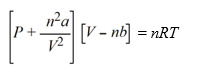 At what pressure will 1.00 mole of CH4 be in a 10.0 L container at 298 K, assuming that CH4 is a real gas?(van der Waals constants for CH4 are a = 2.253 L2 atm mol-2, b = 0.04278 L mol-1)
At what pressure will 1.00 mole of CH4 be in a 10.0 L container at 298 K, assuming that CH4 is a real gas?(van der Waals constants for CH4 are a = 2.253 L2 atm mol-2, b = 0.04278 L mol-1)
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following is a condition that must be met for a gas to be considered as "ideal"?
(Multiple Choice)
4.8/5  (26)
(26)
A sealed glass container contains partial pressures of 0.24 atm O2, 0.45 atm CO2, 0.64 atm N2, and 0.46 atm H2 gas in a 3.5 L container at 30.5°C. What is the total pressure exerted on the walls of the container?
(Multiple Choice)
4.7/5  (37)
(37)
A gas sample is attached to a closed end mercury manometer. The mercury on the end of the manometer attached to the gas sample is 170 mm lower than the mercury on the closed end. What is the pressure of the gas in the sample?
(Short Answer)
4.7/5  (35)
(35)
A sample of a gas in a cylindrical chamber with a movable piston occupied a volume of 1.40 liters when the pressure was 762 torr and the temperature was 26.9°C. The volume of the system was readjusted to 0.150 liters by moving the piston. What is the pressure exerted on the surface of the piston by the gas if the temperature of the system remained constant?
(Multiple Choice)
4.8/5  (37)
(37)
A sample of a gas occupies a volume of 122.4 mL at STP. It was placed in a different vessel with a volume of 164.2 mL in which the pressure was measured as 0.9915 atm. What is its temperature in this new vessel?
(Multiple Choice)
4.8/5  (40)
(40)
A sealed glass container contains 0.2 moles of O2 gas and 0.3 moles of N2 gas. If the total pressure inside the container is 0.75 atm what is the partial pressure of O2 in the glass container?
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the density of Br2 gas at 59.0°C and 2.00 atm pressure.
(Short Answer)
4.9/5  (36)
(36)
What is the mole fraction of methane in a gaseous mixture that consists of 8.00 g of methane and 12.00 g of ethane (C2H6)in a 3.50 L container maintained at 35.20 °C?
(Multiple Choice)
4.9/5  (38)
(38)
A pressure measured using a mercury barometer results in a mercury column height of 356 mm. What column height of water would be needed to measure the same pressure? The density of mercury is 13.6 g/cm3; the density of water is 1.00 g/cm3.
(Multiple Choice)
4.8/5  (29)
(29)
A closed-end manometer was constructed from a U-shaped glass tube. It was loaded with mercury so that the closed side was filled all the way to the top, which was 800 mm above the neck, while the open end was at a level 180 mm above the neck. The manometer was taken into a chamber used for training astronauts. What is the highest pressure that can be read with assurance on this manometer?
(Multiple Choice)
4.8/5  (40)
(40)
If container "A"is occupied by 1.00 mole of oxygen gas while container "B"is occupied by 20.0 grams of nitrogen gas and both containers are maintained at 0.00 °C and 650 torr then,
(Multiple Choice)
4.8/5  (29)
(29)
What is the gas pressure of the gas sample hooked up to the mercury manometer shown below if the atmospheric pressure is 756 mmHg? 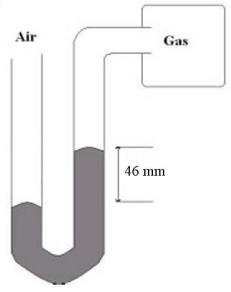
(Short Answer)
4.9/5  (47)
(47)
A gaseous element has a density of 3.195 g L−1 when the temperature is 35.8 °C and the pressure is 734.6 torr. Which element best fits the description?
(Short Answer)
4.8/5  (44)
(44)
Showing 121 - 140 of 162
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)