Exam 11: Properties of Gases
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Which will diffuse more rapidly, C3H8 (propane)or C4H10 (butane)? Why?
(Short Answer)
4.8/5  (31)
(31)
A sample of a gas in a cylindrical chamber with a movable piston occupied a volume of 2.86 cubic meters when the pressure was 9.85 × 104 Pa and the temperature was 25.8°C. The pressure was readjusted to 1.08 × 105 Pa by moving the piston. What is the volume occupied by the sample under the new conditions if the temperature remained constant throughout?
(Multiple Choice)
4.9/5  (36)
(36)
Results of measurements on a gas to determine its molecular mass gave a value of 1.614 g L−1 for the density, at 27.2 °C and 749.4 torr. What is the molecular mass of the gas?
(Short Answer)
4.8/5  (42)
(42)
A sample of a gas was isolated in a gas containment bulb on a manifold used in this type work. The volume of the bulb was 1.524 liters. The temperature was 28.40 °C, and the manifold pressure was 637.6 torr. What volume would this gas sample occupy at STP?
(Multiple Choice)
4.8/5  (38)
(38)
A certain gas behaves as an ideal gas. At a temperature of 25.0 °C and a pressure of 760.0 torr, one mole of this gas occupies a volume of 22.4 liters.
(True/False)
4.9/5  (30)
(30)
A sample of a gas occupies a volume of 1.462 liters at STP. It was placed in a different vessel in which the pressure was measured as 722.5 torr when the temperature was 25.20 °C. What is the volume of this new vessel?
(Multiple Choice)
4.9/5  (41)
(41)
The average speed at which a nitrogen molecule effuses at 30.0 °C is 480 meters per second. The average speed at which a butene molecule (C4H8)effuses at this same temperature should therefore be
(Multiple Choice)
4.8/5  (38)
(38)
How many liters of pure oxygen gas, measured at STP, are required for the complete combustion of 16.6 L of ethylene gas (C2H4), also measured at STP?
(Multiple Choice)
4.8/5  (38)
(38)
A gas sample occupies a volume of 1.264 L when the temperature is 168.0 °C and the pressure is 946.6 torr. How many molecules are in the sample?
(Multiple Choice)
4.9/5  (27)
(27)
The van der Waals equation of state for a real gas is 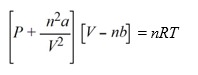 At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
(Multiple Choice)
4.8/5  (34)
(34)
Gases can have negative volumes when the temperature falls below 0°C.
(True/False)
5.0/5  (45)
(45)
A gas sample containing 0.2820 moles of a compound is trapped in a 2.461 liter vessel at a temperature of 25.2 °C. What is the pressure in the vessel if it behaves as an ideal gas?
(Multiple Choice)
4.9/5  (41)
(41)
What is the total pressure exerted by a gaseous mixture that consists of 1.00 g of hydrogen and 8.00 g of neon in a 2.80 L container maintained at 44.10 °C?
(Multiple Choice)
4.9/5  (35)
(35)
A sealed glass container contains partial pressures of 0.80 atm CO2 gas and 0.35 atm N2 gas. What is the mole fraction of N2 in the glass container?
(Multiple Choice)
4.8/5  (36)
(36)
A sample of a gas occupies a volume of 1.820 liters at STP. What pressure would it exert if transferred to a 1.425-liter vessel in which its temperature was raised to 25.2 °C?
(Multiple Choice)
4.8/5  (32)
(32)
A sample of nitrogen gas was collected by displacement of water in a gas collection flask. The total pressure in the collection flask was measured as 754.2 torr, the temperature was 20.0 °C, and the measured volume of gas collected was 516 mL. How many grams should the nitrogen weigh? (At 20.0 °C, the vapor pressure of water is 17.5 torr.)
(Multiple Choice)
4.8/5  (41)
(41)
A sample of hydrogen gas was collected by displacement of water in a large gas buret. The total pressure in the buret was measured as 764.2 torr, the temperature was 23.0 °C, and the buret contained 511 mL of the collected gas. How many moles of hydrogen were collected?(At 23.0 °C, the vapor pressure of water is 21.1 torr.)
(Multiple Choice)
4.7/5  (34)
(34)
Complete the following sentence: The molecules of different ideal gas samples have the same average kinetic energies at the same ________.
(Short Answer)
4.8/5  (44)
(44)
During a chemical demonstration, a filled balloon is submerged in liquid nitrogen, an extremely cold material. As the balloon sits in the liquid nitrogen, it appears to deflate. However, when the balloon is removed from the nitrogen, it appears to fill again. What is happening to the gas inside the balloon that gives this visual result?
(Short Answer)
4.8/5  (32)
(32)
Showing 101 - 120 of 162
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)