Exam 3: Structure of the Atom
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Determine the period and group in the periodic chart that contains the element with the following electron configuration:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
(Multiple Choice)
4.7/5  (46)
(46)
What is the maximum number of unpaired electrons that can be accommodated in a 5d subshell?
(Multiple Choice)
4.8/5  (35)
(35)
Arrange the following atoms in order of increasing first ionization energies.
(Multiple Choice)
4.8/5  (44)
(44)
Which element is represented by the following PES spectrum? 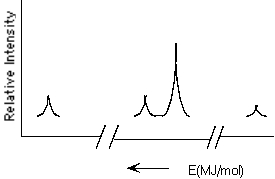
(Multiple Choice)
4.8/5  (39)
(39)
A recent article in Scientific American notes that life would be impossible on any planet close enough to the Sun to boil water, or far enough from the Sun that water froze. It also argues that the Earth is in one of the few parts of the galaxy where life could exist. Too close to the core, and either collisions with other objects would destroy the planet or cosmic radiation from neighboring stars would destroy life. Too far from the sun and there wouldn't be enough of the elements needed to form a planet. Let's assume that radiation becomes particularly dangerous to life when it carries enough energy to ionize a water molecule when it is absorbed. ?
 Use Avogadro's number and Planck's constant to calculate the frequency of this radiation to one significant figure. (Hint: Pay close attention to units!)
Use Avogadro's number and Planck's constant to calculate the frequency of this radiation to one significant figure. (Hint: Pay close attention to units!)
(Multiple Choice)
4.7/5  (39)
(39)
Theoreticians predict that the element with atomic number 120 will be more stable than the elements recently discovered with atomic numbers between 103 and 109. Based on the order of filling of atomic orbitals, the chemistry of this element should most closely resemble the chemistry of which of the following?
(Multiple Choice)
4.9/5  (34)
(34)
Arrange the following elements in order of increasing first ionization energy:
S, Ar, Ca
(Multiple Choice)
4.9/5  (26)
(26)
O2 molecules can dissociate to form O atoms by absorbing electromagnetic radiation. If it takes 498 kJ to dissociate one mole of O2 molecules to form two moles of O atoms, in what portion of the electromagnetic spectrum would light have sufficient energy to cause this reaction to occur?
(Multiple Choice)
4.7/5  (39)
(39)
Which atom contains the largest number of unpaired electrons?
(Multiple Choice)
4.8/5  (28)
(28)
What do we mean when we say that "The energy of the electron in an atom is quantized?"
(Multiple Choice)
4.8/5  (37)
(37)
What is the maximum number of electrons that can be accommodated in the subshell for which n = 3 and l = 2?
(Multiple Choice)
4.8/5  (34)
(34)
Determine the group of the periodic table in which an element with the following electron configuration belongs: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
(Multiple Choice)
4.8/5  (41)
(41)
Arrange the following atoms in order of decreasing energy needed to remove the most tightly held electron (the electron closest to the nucleus).
Na, Mg, Al, P
(Multiple Choice)
4.8/5  (36)
(36)
In 1814 Fraunhofer observed a series of dark lines in the sun's spectrum, which he labeled A through H. About 50 years later, Gustav Kirchhoff noticed that the wavelength of light given off when sodium salts are added to a flame is the same as the wavelength of the D line in Fraunhofer's spectrum. He concluded that certain substances give off light when heated that has the same wavelength as the light absorbed under other conditions. The wavelength of the characteristic yellow-orange light emitted by sodium ions in a burner flame is 589.5923 nm. What is the energy of this light, in units of kJ/mol?
(Multiple Choice)
4.9/5  (36)
(36)
Water ionizes according to the following reaction which requires approximately 1200 kJ/mol of energy
H2O(l) + h v H2O+ + e-
In what portion of the electromagnetic spectrum are you likely to find the radiation that carries just enough energy to be dangerous to life because it can ionize the water that is so important to living organisms?
(Multiple Choice)
4.7/5  (38)
(38)
Which transition shown below will require the absorption of the shortest wavelength photon?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following quantum numbers can have a value that is not an integer?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following describes the electron configuration for the Sn2+ ion?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 41 - 60 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)