Exam 3: Structure of the Atom
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Why is the ionization energy of potassium (K) less than the ionization energy of sodium (Na)?
(Multiple Choice)
4.9/5  (35)
(35)
A photoelectron spectrum has three peaks. The first two are one third the size of the last one. Which atom corresponds to this spectrum?
(Multiple Choice)
4.9/5  (36)
(36)
What would you describe as the key result of the Rutherford experiment, in which a metal target was bombarded with
-particles?
(Multiple Choice)
4.9/5  (38)
(38)
A helium-neon laser emits light with a wavelength of 6328 Å. What is the energy of a 6328 Å photon in joules?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements about the Bohr model is wrong?
(Multiple Choice)
4.9/5  (28)
(28)
Which element is represented by the following PES spectrum?
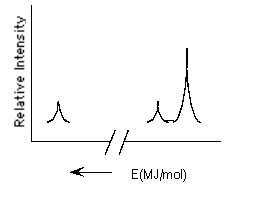
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following transitions in the spectrum of the hydrogen atom results in the absorption of a photon with the largest energy?
(Multiple Choice)
4.9/5  (31)
(31)
In what group of the periodic table would an element with the following electron configuration belong?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p1
(Multiple Choice)
4.8/5  (38)
(38)
A possible set of quantum numbers for the last electron added to form a gallium atom (Z = 31) in its lowest energy state is:
(Multiple Choice)
4.9/5  (44)
(44)
Which set of quantum numbers can be used to describe a 2p electron?
(Multiple Choice)
4.8/5  (36)
(36)
Arrange the following atoms in order of increasing first ionization energy.
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following selection rules for quantum numbers is incorrectly stated?
(Multiple Choice)
4.9/5  (29)
(29)
Why is the first ionization energy of Br less than that of Cl?
(Multiple Choice)
4.8/5  (31)
(31)
Which transition in the spectrum of the hydrogen atom results in the emission of light with the longest wavelength?
(Multiple Choice)
4.7/5  (35)
(35)
In which of the following groups of atoms does each atom have the same number of outer shell electrons?
(Multiple Choice)
4.9/5  (41)
(41)
In period 6 of the periodic table, the series of elements which differ in the number of electrons contained in f orbitals
(Multiple Choice)
4.9/5  (37)
(37)
Showing 61 - 80 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)