Exam 3: Structure of the Atom
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
The outermost or highest energy electron in element 105 could be characterized by which of the following sets of n, l, ml and ms quantum numbers?
(Multiple Choice)
4.8/5  (30)
(30)
Which atom is represented by a shell model with two electrons in the first shell, and five electrons in the second shell?
(Multiple Choice)
4.9/5  (42)
(42)
What is the value of x in the following electron configuration for silicon?
1s2 2s2 2p6 3s2 3px
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following sets of n, l, ml and ms quantum numbers isn't allowed?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following subshells is filled first when electrons are added to the atomic orbitals on a xenon atom?
(Multiple Choice)
4.9/5  (31)
(31)
The first ionization energy of the first six elements in the periodic table is given below.
 Why is the first ionization energy of B smaller than Be?
Why is the first ionization energy of B smaller than Be?
(Multiple Choice)
4.9/5  (33)
(33)
The line structure of the emission spectrum of the hydrogen atom suggests that:
(Multiple Choice)
4.9/5  (46)
(46)
If X-rays have a shorter wavelength than ultraviolet light, which of the following statements is true?
(Multiple Choice)
4.8/5  (36)
(36)
Give the orbital diagram (using parentheses and arrows to represent orbitals and electrons) of phosphorus.
(Essay)
4.9/5  (39)
(39)
For each pair of atoms, which has the smallest metallic radius?
A) Na, Be
B) K, Mg
C) Cs, Ra
(Short Answer)
5.0/5  (30)
(30)
What is the lowest energy electronic configuration of a fluorine atom?
(Multiple Choice)
4.9/5  (32)
(32)
What is the orbital designation for the quantum numbers:
N = 4, l = 2, ml = -1?
(Multiple Choice)
4.8/5  (46)
(46)
Which is a legitimate set of n, l, ml and ms quantum numbers?
(Multiple Choice)
4.7/5  (43)
(43)
Which element is represented by the following PES spectrum?
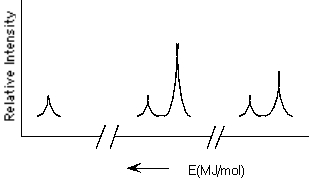
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following atoms would have four peaks in its PES spectrum?
(Multiple Choice)
4.8/5  (43)
(43)
A possible set of quantum numbers for the highest energy electron in an As3+ ion is:
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following quantum numbers is used to describe the orientation of an orbital in space?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)