Exam 27: Quantum Physics
Exam 1: Measurement and Problem Solving98 Questions
Exam 2: Kinematics: Description of Motion92 Questions
Exam 3: Motion in Two Dimensions104 Questions
Exam 4: Force and Motion64 Questions
Exam 5: Work and Energy62 Questions
Exam 6: Linear Momentum and Collisions71 Questions
Exam 7: Circular Motion and Gravitation93 Questions
Exam 8: Rotational Motion and Equilibrium68 Questions
Exam 9: Solids and Fluids75 Questions
Exam 10: Temperature and Kinetic Theory68 Questions
Exam 11: Heat61 Questions
Exam 12: Thermodynamics70 Questions
Exam 13: Vibrations and Waves79 Questions
Exam 14: Sound61 Questions
Exam 15: Electric Charge, Forces, and Fields46 Questions
Exam 16: Electric Potential, Energy, and Capacitance47 Questions
Exam 17: Electric Current and Resistance59 Questions
Exam 18: Basic Electric Circuits68 Questions
Exam 19: Magnetism58 Questions
Exam 20: Electromagnetic Induction and Waves43 Questions
Exam 21: AC Circuits50 Questions
Exam 22: Reflection and Refraction of Light53 Questions
Exam 23: Mirrors and Lenses61 Questions
Exam 24: Physical Optics: The Wave Nature of Light57 Questions
Exam 25: Vision and Optical Instruments53 Questions
Exam 26: Relativity60 Questions
Exam 27: Quantum Physics58 Questions
Exam 28: Quantum Mechanics and Atomic Physics54 Questions
Exam 29: The Nucleus63 Questions
Exam 30: Nuclear Reactions and Elementary Particles54 Questions
Select questions type
In the Compton effect, as the scattering angle increases, the frequency of the X-rays scattered at that angle
(Multiple Choice)
4.7/5  (37)
(37)
Give an example of a material whose useful property depends upon a metastable state.
(Short Answer)
4.9/5  (43)
(43)
The graph shown in Figure 27-1 is a plot based on student data from their testing of a
photoelectric material.
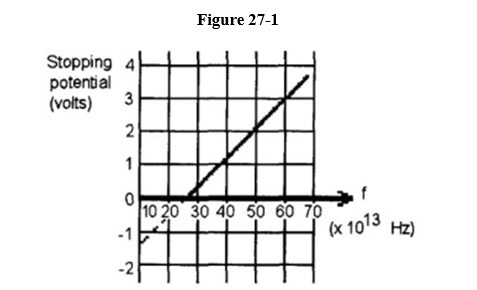 (a) Determine the cutoff frequency.
(b) Determine the work function.
(a) Determine the cutoff frequency.
(b) Determine the work function.
(Short Answer)
4.8/5  (41)
(41)
When the accelerating voltage in an X-ray tube is doubled, the minimum wavelength of the X-rays
(Multiple Choice)
4.8/5  (35)
(35)
Bohr's atomic theory predicted energy levels for hydrogen of En = -(2 π2k2e4m) about a bare nucleus of atomic number Z, how do you expect Z to be incorporated into the En formula and why?
(Essay)
5.0/5  (38)
(38)
What is the ionization energy for a doubly ionized lithium ion (Z = 3)?
(Short Answer)
4.7/5  (45)
(45)
Classical theory predicts that the planets can orbit the Sun quite stably. Why could not stable orbits be predicted for the atom by simply substituting the electrical attraction for the gravitational attraction? Why was there a classical crisis in describing the atom?
(Essay)
4.9/5  (50)
(50)
You may have heard it said that all objects, even ones at the temperature of our bodies, are continually emitting electromagnetic radiation. Is this true?
(Multiple Choice)
4.9/5  (42)
(42)
Which hydrogen spectral series falls in the visible region?
(Multiple Choice)
4.7/5  (40)
(40)
In the Bohr theory the orbital radius depends upon the principal quantum number in what way?
(Multiple Choice)
4.8/5  (39)
(39)
When an electron jumps from an orbit where n = 4 to one where n = 3
(Multiple Choice)
4.9/5  (39)
(39)
Consider an atom with four accessible energy levels. What is the maximum number of different wavelengths that could be emitted by such an atom?
(Multiple Choice)
4.8/5  (43)
(43)
In a Compton scattering experiment, what scattering angle produces the greatest change in wavelength?
(Multiple Choice)
4.8/5  (40)
(40)
The Compton effect importantly demonstrated which property of electromagnetic radiation?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 21 - 40 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)