Exam 8: An Introduction to Metabolism
Exam 1: Introduction: Themes in the Study of Life66 Questions
Exam 2: The Chemical Context of Life83 Questions
Exam 3: Water and the Fitness of the Environment66 Questions
Exam 4: Carbon and the Molecular Diversity of Life68 Questions
Exam 5: The Structure and Function of Large Biological Molecules109 Questions
Exam 6: A Tour of the Cell75 Questions
Exam 7: Membrane Structure and Function75 Questions
Exam 8: An Introduction to Metabolism79 Questions
Exam 9: Cellular Respiration: Harvesting Chemical Energy103 Questions
Exam 10: Photosynthesis74 Questions
Exam 11: Cell Communication62 Questions
Exam 12: The Cell Cycle80 Questions
Exam 13: Meiosis and Sexual Life Cycles68 Questions
Exam 14: Mendel and the Gene Idea90 Questions
Exam 15: The Chromosomal Basis of Inheritance75 Questions
Exam 16: The Molecular Basis of Inheritance72 Questions
Exam 17: From Gene to Protein84 Questions
Exam 18: Control of Gene Expression101 Questions
Exam 19: Viruses38 Questions
Exam 20: Biotechnology70 Questions
Exam 21: Genomes and Their Evolution37 Questions
Exam 22: Descent With Modification: a Darwinian View of Life57 Questions
Exam 23: The Evolution of Populations84 Questions
Exam 24: The Origin of Species60 Questions
Exam 25: The History of Life on Earth85 Questions
Exam 26: Phylogeny and the Tree of Life90 Questions
Exam 27: Bacteria and Archaea78 Questions
Exam 28: Protists79 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land74 Questions
Exam 30: Plant Diversity Ii: the Evolution of Seed Plants101 Questions
Exam 31: Fungi87 Questions
Exam 32: An Introduction to Animal Diversity82 Questions
Exam 33: Invertebrates98 Questions
Exam 34: Vertebrates112 Questions
Exam 35: Plant Structure, Growth, and Development77 Questions
Exam 36: Transport in Vascular Plants84 Questions
Exam 37: Soil and Plant Nutrition85 Questions
Exam 38: Angiosperm Reproduction and Biotechnology86 Questions
Exam 39: Plant Responses to Internal and External Signals111 Questions
Exam 40: Basic Principles of Animal Form and Function74 Questions
Exam 41: Animal Nutrition68 Questions
Exam 42: Circulation and Gas Exchange78 Questions
Exam 43: The Immune System85 Questions
Exam 44: Osmoregulation and Excretion49 Questions
Exam 45: Hormones and the Endocrine System71 Questions
Exam 46: Animal Reproduction85 Questions
Exam 47: Animal Development75 Questions
Exam 48: Neurons, Synapses, and Signaling52 Questions
Exam 49: Nervous Systems48 Questions
Exam 50: Sensory and Motor Mechanisms59 Questions
Exam 51: Animal Behavior74 Questions
Exam 52: An Introduction to Ecology and the Biosphere71 Questions
Exam 53: Population Ecology80 Questions
Exam 54: Community Ecology74 Questions
Exam 55: Ecosystems79 Questions
Exam 56: Conservation Biology and Restoration Ecology65 Questions
Select questions type
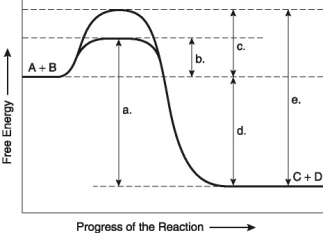
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is known as
-The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is known as
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Which of the following shows the correct changes in thermodynamic properties for a chemical reaction in which amino acids are linked to form a protein?
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
C
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -Which of the following represents the activation energy required for the enzyme-catalyzed reaction?
-Which of the following represents the activation energy required for the enzyme-catalyzed reaction?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
Which of the following statements regarding enzymes is True?
(Multiple Choice)
4.8/5  (39)
(39)
What term is used to describe the transfer of free energy from catabolic pathways to anabolic pathways?
(Multiple Choice)
4.9/5  (33)
(33)
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -Assume that the reaction has a △G of -5.6 kcal/mol. Which of the following would be True?
-Assume that the reaction has a △G of -5.6 kcal/mol. Which of the following would be True?
(Multiple Choice)
4.7/5  (41)
(41)
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -Which of the following represents the difference between the free-energy content of the reaction and the free-energy content of the products?
-Which of the following represents the difference between the free-energy content of the reaction and the free-energy content of the products?
(Multiple Choice)
4.8/5  (48)
(48)
Chemical equilibrium is relatively rare in living cells. Which of the following could be an example of a reaction at chemical equilibrium in a cell?
(Multiple Choice)
4.8/5  (33)
(33)
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -In order to attach a particular amino acid to the tRNA molecule that will transport it, an enzyme, an aminoacyl-tRNA synthetase, is required, along with ATP. Initially, the enzyme has an active site for ATP and another for the amino acid, but it is not able to attach the tRNA. What must occur in order for the final attachment to occur?
-In order to attach a particular amino acid to the tRNA molecule that will transport it, an enzyme, an aminoacyl-tRNA synthetase, is required, along with ATP. Initially, the enzyme has an active site for ATP and another for the amino acid, but it is not able to attach the tRNA. What must occur in order for the final attachment to occur?
(Multiple Choice)
4.7/5  (36)
(36)
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -Which of the following is likely to lead to an increase in the concentration of ATP in a cell?
-Which of the following is likely to lead to an increase in the concentration of ATP in a cell?
(Multiple Choice)
4.9/5  (34)
(34)
For living organisms, which of the following is an important consequence of the first law of thermodynamics?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following metabolic processes can occur without a net influx of energy from some other process?
(Multiple Choice)
4.8/5  (45)
(45)
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
 -What is substance X?
-What is substance X?
(Multiple Choice)
4.8/5  (37)
(37)
Use the following information to answer the following questions.
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
-What is the purpose of using malonic acid in this experiment?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is True for all exergonic reactions?
(Multiple Choice)
4.9/5  (26)
(26)
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
(Multiple Choice)
4.7/5  (32)
(32)
Whenever energy is transformed, there is always an increase in the
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is a logical consequence of the second law of thermodynamics?
(Multiple Choice)
4.8/5  (38)
(38)
The mathematical expression for the change in free energy of a system is △G =△H-T△S. Which of the following is (are)correct?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)