Exam 7: Chemical Quantities and Reactions
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
In the following reaction,when 100.g of Br2 reacts,111 g of AlBr3 is formed.
2Al + 3 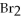 → 2
→ 2 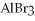
(True/False)
4.8/5  (33)
(33)
The reaction of carbon with oxygen to produce carbon monoxide is an example of which class of reaction?
2C + O2 → 2CO
(Multiple Choice)
4.9/5  (33)
(33)
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
-In this reaction,what is the correct coefficient for hydrogen gas?
? H2 + ? O2 → ? H2O
(Multiple Choice)
4.9/5  (37)
(37)
For the following question(s),consider the following equation.
2Mg + 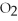 → 2MgO
-The number of moles of MgO produced when 0.20 mole of
→ 2MgO
-The number of moles of MgO produced when 0.20 mole of 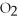 reacts completely is ________.
reacts completely is ________.
(Multiple Choice)
4.8/5  (34)
(34)
If the reaction shown below is exothermic,the energy level of the reactants is ________.
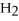 +
+ 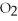 →
→ 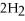 O
O
(Multiple Choice)
4.8/5  (30)
(30)
For the following question(s),consider the following balanced equation.
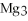
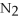 + 6
+ 6 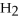 O → 3
O → 3 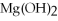 + 2
+ 2 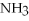 -When 4 moles of aluminum are allowed to react with an excess of chlorine gas,
-When 4 moles of aluminum are allowed to react with an excess of chlorine gas, 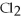 ,how many moles of aluminum chloride are produced?
2Al + 3
,how many moles of aluminum chloride are produced?
2Al + 3 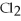 → 2Al
→ 2Al 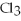
(Multiple Choice)
4.8/5  (40)
(40)
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
-How many moles of barium sulfate are produced from 0.1 mole of barium chloride?
(Multiple Choice)
4.9/5  (40)
(40)
For the following question(s),consider the following equation.
2Mg + 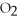 → 2MgO
-In the reaction of silver nitrate with sodium chloride,how many grams of silver chloride will be produced from 100.g of silver nitrate when it is mixed with an excess of sodium chloride? The equation for the reaction is below.
AgN
→ 2MgO
-In the reaction of silver nitrate with sodium chloride,how many grams of silver chloride will be produced from 100.g of silver nitrate when it is mixed with an excess of sodium chloride? The equation for the reaction is below.
AgN 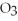 + NaCl → AgCl + NaN
+ NaCl → AgCl + NaN 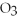
(Multiple Choice)
4.9/5  (30)
(30)
The number of particles in 1 mole of hydrogen gas is ________.
(Short Answer)
4.8/5  (37)
(37)
In this reaction,what is the substance oxidized?
Zn + 2 HCl → ZnCl2 + H2
(Multiple Choice)
5.0/5  (43)
(43)
Is the following reaction exothermic or endothermic?
C2H6 + O2 → CO2 + 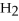 O +1300 kJ
O +1300 kJ
(Short Answer)
4.8/5  (36)
(36)
Which of the following correctly gives the correct coefficients for the reaction below?
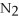
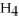 +
+ 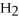
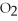 →
→ 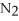 +
+ 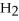 O
O
(Multiple Choice)
5.0/5  (40)
(40)
Showing 61 - 80 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)