Exam 7: Chemical Quantities and Reactions
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
0.400 mole of sucrose, C12H22O11,contains ________ C atoms.
(Multiple Choice)
4.8/5  (35)
(35)
What is oxidized and what is reduced in the following reaction?
2Al + 3 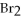 → 2
→ 2 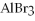
(Multiple Choice)
4.8/5  (39)
(39)
For the following question(s),consider the following balanced equation.
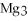
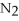 + 6
+ 6 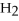 O → 3
O → 3 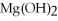 + 2
+ 2 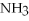 -How many grams of NO are required to produce 145 g of
-How many grams of NO are required to produce 145 g of 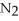 in the following reaction?
4
in the following reaction?
4 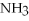 + 6NO → 5
+ 6NO → 5 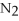 + 6
+ 6 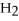 O
O
(Multiple Choice)
4.8/5  (39)
(39)
In this reaction,what is the coefficient for calcium oxide?
CaO + CO2 → CaCO3
(Multiple Choice)
4.9/5  (40)
(40)
Pentane ( 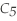
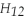 )reacts with oxygen (
)reacts with oxygen ( 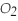 )to form carbon dioxide (
)to form carbon dioxide ( 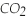 )and water (
)and water ( 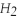 O)according to the following reaction.Answer the following question(s)about this reaction.
O)according to the following reaction.Answer the following question(s)about this reaction. 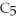
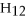 + ?
+ ? 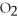 → ?
→ ? 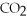 + ?
+ ? 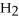 O
-What is the coefficient for carbon dioxide in the balanced equation?
O
-What is the coefficient for carbon dioxide in the balanced equation?
(Multiple Choice)
4.9/5  (41)
(41)
In the following reaction,what is the correct coefficient for aluminum chloride?
Al + 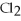 → Al
→ Al 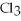
(Multiple Choice)
4.8/5  (33)
(33)
What is the classification for this unbalanced reaction?
Fe + HCl → FeC 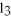 + H2
+ H2
(Multiple Choice)
4.9/5  (40)
(40)
When 25.0 g of KCl 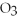 reacts,how many grams of O2 are produced?
2KCl
reacts,how many grams of O2 are produced?
2KCl 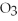 → 2KCl + 3
→ 2KCl + 3 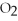
(Short Answer)
4.9/5  (32)
(32)
For the following question(s),consider the following equation.
2Mg + 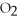 → 2MgO
-How many grams of magnesium are needed to react with 16 g of
→ 2MgO
-How many grams of magnesium are needed to react with 16 g of 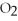 ?
?
(Multiple Choice)
4.8/5  (43)
(43)
Pentane ( 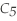
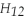 )reacts with oxygen (
)reacts with oxygen ( 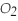 )to form carbon dioxide (
)to form carbon dioxide ( 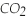 )and water (
)and water ( 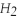 O)according to the following reaction.Answer the following question(s)about this reaction.
O)according to the following reaction.Answer the following question(s)about this reaction. 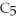
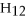 + ?
+ ? 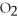 → ?
→ ? 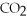 + ?
+ ? 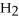 O
-What is the coefficient for oxygen in the balanced equation?
O
-What is the coefficient for oxygen in the balanced equation?
(Multiple Choice)
4.8/5  (37)
(37)
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
-How many grams of barium chloride are needed to make 100.grams of barium sulfate?
(Multiple Choice)
4.9/5  (34)
(34)
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
-How many grams of sodium chloride can be produced from 50.grams of barium chloride?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)