Exam 7: Chemical Quantities and Reactions
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
What coefficient is placed in front of O2 to complete the balancing of the following equation?
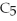
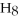 + ?
+ ? 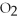 → 5
→ 5 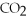 + 4 H2O
+ 4 H2O
(Multiple Choice)
4.8/5  (41)
(41)
In an oxidation-reduction reaction,the substance oxidized always ________.
(Multiple Choice)
4.9/5  (30)
(30)
Answer the question(s)below about the following reaction.
2 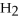
 → 2
→ 2 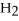 O +
O + 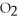 -How many grams of hydrogen peroxide are needed to produce 25.0 g of oxygen?
-How many grams of hydrogen peroxide are needed to produce 25.0 g of oxygen?
(Multiple Choice)
4.8/5  (37)
(37)
For the following question(s),consider the following balanced equation.
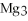
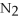 + 6
+ 6 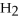 O → 3
O → 3 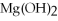 + 2
+ 2 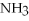 -In the reaction of nitrogen gas,
-In the reaction of nitrogen gas, 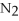 ,with hydrogen gas,
,with hydrogen gas, 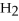 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen?
,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen?
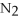 + 3
+ 3 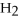 → 2
→ 2 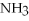
(Multiple Choice)
4.9/5  (38)
(38)
When the following equation is balanced,the coefficient of O2 is ________.
CH4 + O2 → CO2 + 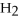 O
O
(Short Answer)
4.8/5  (38)
(38)
Which of the following gives the balanced equation for this reaction?
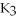
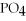 + Ca
+ Ca 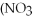
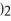 →
→ 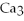
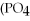
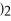 +
+ 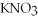
(Multiple Choice)
4.8/5  (32)
(32)
When the following equation is balanced,the coefficient of O2 is ________.
C2H6 + O2 → CO2 + 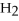 O
O
(Short Answer)
4.9/5  (37)
(37)
Pentane ( 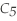
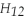 )reacts with oxygen (
)reacts with oxygen ( 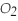 )to form carbon dioxide (
)to form carbon dioxide ( 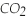 )and water (
)and water ( 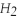 O)according to the following reaction.Answer the following question(s)about this reaction.
O)according to the following reaction.Answer the following question(s)about this reaction. 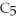
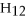 + ?
+ ? 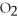 → ?
→ ? 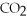 + ?
+ ? 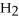 O
-What is the coefficient for water in the balanced equation?
O
-What is the coefficient for water in the balanced equation?
(Multiple Choice)
4.9/5  (36)
(36)
Answer the question(s)below about the following reaction.
2 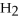
 → 2
→ 2 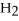 O +
O + 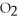 -How many moles of hydrogen peroxide (H2O2)are needed to produce 5.0 moles of water?
-How many moles of hydrogen peroxide (H2O2)are needed to produce 5.0 moles of water?
(Multiple Choice)
4.8/5  (42)
(42)
Answer the question(s)below about the following reaction.
2 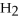
 → 2
→ 2 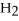 O +
O + 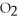 -This is an example of a ________ reaction.
-This is an example of a ________ reaction.
(Multiple Choice)
4.8/5  (30)
(30)
For the following question(s),consider the following equation.
2Mg + 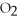 → 2MgO
-How many grams of hydrogen are needed to produce 1.80 g of water according to this equation?
2
→ 2MgO
-How many grams of hydrogen are needed to produce 1.80 g of water according to this equation?
2 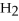 +
+ 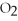 → 2
→ 2 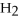 O
O
(Multiple Choice)
4.8/5  (40)
(40)
For the following question(s),consider the following balanced equation.
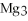
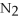 + 6
+ 6 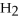 O → 3
O → 3 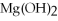 + 2
+ 2 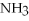 -What is the correct form of the conversion factor needed to convert the number of moles of
-What is the correct form of the conversion factor needed to convert the number of moles of 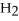 O to the number of moles of
O to the number of moles of 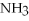 produced?
produced?
(Multiple Choice)
4.8/5  (41)
(41)
In an oxidation-reduction reaction,the substance reduced always ________.
(Multiple Choice)
4.9/5  (40)
(40)
In a ________ reaction,two or more elements or compounds form one product.
(Multiple Choice)
4.8/5  (40)
(40)
Given the following equation,what is the correct form of the conversion factor needed to convert the number of moles of 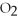 to the number of moles of
to the number of moles of 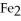
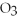 produced?
4Fe + 3
produced?
4Fe + 3 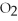 → 2
→ 2 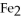
(Multiple Choice)
4.9/5  (42)
(42)
Showing 81 - 100 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)