Exam 7: Chemical Quantities and Reactions
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
For the following question(s),consider the following equation.
2Mg + 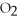 → 2MgO
-How many grams of MgO are produced when 40.0 grams of
→ 2MgO
-How many grams of MgO are produced when 40.0 grams of 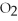 react completely with Mg?
react completely with Mg?
(Multiple Choice)
4.8/5  (32)
(32)
For the following question(s),consider the following balanced equation.
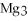
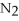 + 6
+ 6 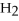 O → 3
O → 3 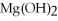 + 2
+ 2 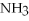 -How many grams of
-How many grams of 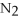 are produced when 125 g of
are produced when 125 g of 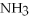 reacts as shown in the following balanced equation?
4
reacts as shown in the following balanced equation?
4 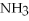 + 6NO → 5
+ 6NO → 5 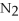 + 6
+ 6 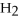 O
O
(Multiple Choice)
4.8/5  (40)
(40)
The following reaction is endothermic.
2 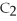
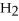 + 5
+ 5 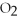 → 4C
→ 4C 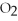 +
+ 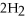 O + 310 kcal
O + 310 kcal
(True/False)
4.8/5  (33)
(33)
The ________ is the minimum energy needed for a chemical reaction to begin.
(Multiple Choice)
4.9/5  (31)
(31)
For the following question(s),consider the following balanced equation.
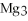
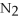 + 6
+ 6 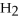 O → 3
O → 3 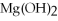 + 2
+ 2 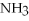 -When 2 moles of
-When 2 moles of 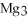
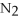 are allowed to react,how many moles of
are allowed to react,how many moles of 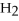 O also react?
O also react?
(Multiple Choice)
4.7/5  (46)
(46)
The following reaction takes place when an electric current is passed through water.It is an example of a ________ reaction.
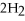 O
O 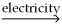 2
2 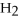 +
+ 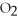
(Multiple Choice)
4.7/5  (38)
(38)
Acetylene gas, 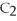
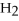 ,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
2
,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
2 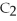
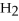 + 5
+ 5 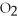 → 4C
→ 4C 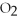 +
+ 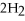 O
O
(Short Answer)
4.9/5  (38)
(38)
Answer the question(s)below about the following reaction.
2 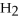
 → 2
→ 2 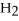 O +
O + 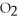 -How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2)produce,if decomposition is complete?
-How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2)produce,if decomposition is complete?
(Multiple Choice)
4.8/5  (33)
(33)
The ________ is the energy difference between reactants and products in a chemical reaction.
(Multiple Choice)
4.7/5  (34)
(34)
What is the molar mass of 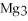
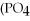
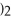 ,a substance formerly used in medicine as an antacid?
,a substance formerly used in medicine as an antacid?
(Multiple Choice)
4.8/5  (27)
(27)
A reaction that releases energy as it occurs is classified as a(n)________.
(Multiple Choice)
4.7/5  (37)
(37)
In the reaction of hydrogen gas with oxygen gas to produce water,1 mole of oxygen gas can produce 2 moles of water,given sufficient hydrogen available.
(True/False)
4.7/5  (42)
(42)
Showing 41 - 60 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)