Exam 12: Intermolecular Forces and Liquids and Solids
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Indicate all the types of intermolecular forces of attraction in C2H6(g).
(Short Answer)
4.9/5  (40)
(40)
Octane, C8H18, boils at 125°C as compared to water, which boils at 100°C. This information suggests that the dispersion forces in nonpolar octane molecules are stronger than dispersion forces and hydrogen bonding in water.
(True/False)
4.8/5  (31)
(31)
Which is expected to have a higher boiling point, C5H12 or C(CH3)4?
(Short Answer)
4.8/5  (35)
(35)
Ethanol (C2H5 - OH)will have a greater viscosity than ethylene glycol (HO - CH2CH2 - OH)at the same temperature.
(True/False)
4.9/5  (36)
(36)
Each of the following substances is a liquid at -50°C. Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3), propane (C3H8), and ethanol (CH3CH2OH).
(Multiple Choice)
4.8/5  (46)
(46)
How much energy (heat)is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? specific heat of ice: 2.09 J/g·°C Hfus = 6.02 kJ/mol
Specific heat of water: 4.18 J/g·°C Hvap = 40.7 kJ/mol
Specific heat of steam: 1.84 J/g·°C
(Multiple Choice)
4.9/5  (33)
(33)
What mass of water would need to evaporate from your skin in order to dissipate 1.7 * 105 J of heat from your body? H2O(l) H2O(g) Hvap = 40.7 kJ/mol
(Multiple Choice)
4.9/5  (34)
(34)
Of the given pair of compounds, which would have the higher boiling point?
H2Se or H2O
(Short Answer)
4.8/5  (42)
(42)
Which one of the following substances is expected to have the highest boiling point?
(Multiple Choice)
4.9/5  (34)
(34)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 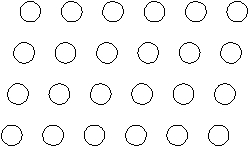 How many atoms are in one unit cell?
How many atoms are in one unit cell?
(Essay)
4.9/5  (37)
(37)
Indicate all the types of intermolecular forces of attraction in SO2(l).
(Short Answer)
4.7/5  (38)
(38)
Which one of the following substances is expected to have the lowest melting point?
(Multiple Choice)
4.9/5  (36)
(36)
Identify the dominant (strongest)type of intermolecular force present in NH3(l).
(Short Answer)
4.9/5  (37)
(37)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
HF or HCl
(Short Answer)
4.9/5  (37)
(37)
Each of the following substances is a gas at 25°C and 1 atmosphere pressure. Which one will liquefy most easily when compressed at a constant temperature?
(Multiple Choice)
4.7/5  (39)
(39)
Given that the heat of vaporization of mercury is 59.0 kJ/mol and the vapor pressure of mercury is 0.0017 torr at 25°C, calculate the normal boiling point of mercury.
(Short Answer)
4.8/5  (42)
(42)
Showing 21 - 40 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)