Exam 12: Intermolecular Forces and Liquids and Solids
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
The shape of the water-to-glass meniscus results from the strong adhesive forces between glass and water.
(True/False)
4.8/5  (40)
(40)
Osmium tetroxide, OsO4, is a soft crystal that melts at 40°C. The liquid does not conduct electricity. What kind of crystal is this?
(Short Answer)
4.8/5  (34)
(34)
Solid iodine has a vapor pressure of 1.0 mmHg at 39°C. How many moles of iodine will sublime into a 500. mL flask at this temperature? If the volume of the flask is doubled at constant temperature, what will happen to the equilibrium vapor pressure of I2? (Assume some solid I2 is always present in the container.)
(Multiple Choice)
4.9/5  (39)
(39)
Given the following compound and its boiling point, identify whether it is polar or nonpolar: H2S, -60.7°C.
(Short Answer)
4.9/5  (43)
(43)
Indicate all the types of intermolecular forces of attraction in CH2O(g).
(Short Answer)
4.9/5  (39)
(39)
Choose the response that lists the member of each of the following pairs that has the higher boiling point. (I)H2O or KI (II)HF or HI (III)Cl2 or Br2
(Multiple Choice)
4.9/5  (43)
(43)
Which one of the following substances will have both dispersion forces and dipole-dipole forces?
(Multiple Choice)
4.8/5  (37)
(37)
Which liquid is expected to have the larger surface tension at a given temperature, CCl4 or H2O? Briefly explain.
(Essay)
4.8/5  (38)
(38)
Identify the dominant (strongest)type of intermolecular force present in H2S(g).
(Short Answer)
4.9/5  (29)
(29)
The normal boiling point of methanol (CH3OH)is 64.6°C. Given that the vapor pressure of methanol is 75.0 torr at 15.2°C, calculate the molar enthalpy of vaporization of methanol.
(Multiple Choice)
4.9/5  (42)
(42)
The freezing point of a liquid does not change as the atmospheric pressure changes.
(True/False)
4.7/5  (40)
(40)
How much enthalpy is necessary to heat 10.0 g of solid benzene (C6H6)at 0.0°C to benzene vapor at 100°C? 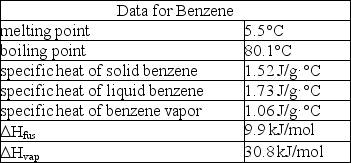
(Short Answer)
4.8/5  (39)
(39)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
SF4 or C10H22
(Short Answer)
4.7/5  (36)
(36)
Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm3 at 27°C. Calculate the atomic radius of Pd.
(Multiple Choice)
4.7/5  (33)
(33)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
H2S or H2Se
(Short Answer)
4.9/5  (30)
(30)
Which of the responses includes all of the following that can form hydrogen bonds with water molecules? (1)Na+ (2)CH3COOH (3)C2H6 (4)CH3NH2
(Multiple Choice)
4.8/5  (42)
(42)
Based on the phase diagram shown below, which is more dense: the liquid phase or the solid phase? 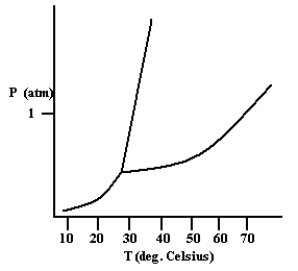
(Short Answer)
4.8/5  (34)
(34)
Helium atoms do not combine to form He2 molecules, yet He atoms do attract one another weakly through
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following characteristics indicates the presence of weak intermolecular forces in a liquid?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 101 - 120 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)