Exam 12: Intermolecular Forces and Liquids and Solids
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Potassium crystallizes in a body-centered cubic lattice. How many atoms are there per unit cell?
(Multiple Choice)
4.8/5  (32)
(32)
Of the given pair of compounds, which would have the higher boiling point?
CH3Cl or CH4
(Short Answer)
4.7/5  (43)
(43)
Which of the following properties indicates the presence of weak intermolecular forces in a liquid?
(Multiple Choice)
4.9/5  (30)
(30)
Use the graph of vapor pressure to determine the normal boiling point of O2. 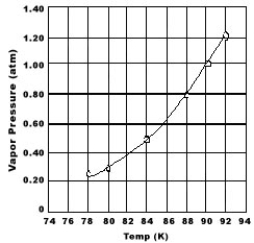
(Multiple Choice)
4.7/5  (41)
(41)
Find the temperature at which water boils on a day in the mountains when the barometric pressure is 593 mmHg. (Given: the heat of vaporization of water is 40.79 kJ/mol)
(Multiple Choice)
4.9/5  (35)
(35)
The triple point of iodine is at 0.12 atm and 115°C. Thus, liquid I2
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following liquids would have the highest viscosity at 25°C?
(Multiple Choice)
4.7/5  (29)
(29)
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3. What is the radius of the platinum atom?
(Multiple Choice)
4.9/5  (32)
(32)
The molar heats of sublimation and fusion of iodine are 62.3 kJ/mol and15.3 kJ/mol, respectively. Calculate the molar heat of vaporization of liquid iodine.
(Multiple Choice)
4.9/5  (41)
(41)
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 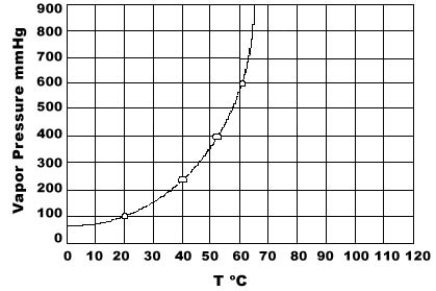
(Multiple Choice)
4.8/5  (29)
(29)
Given that the heat of vaporization of diethyl ether is 26.0 kJ/mol and the vapor pressure of diethyl ether is 440 torr at 20.°C, calculate the normal boiling point of diethyl ether.
(Short Answer)
4.9/5  (36)
(36)
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point?
(Multiple Choice)
4.7/5  (48)
(48)
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg. 400 mmHg @ 104°C, 500 mmHg @ 111°C, 600 mmHg @ 117°C,
700 mmHg @ 122°C, 760 mmHg @ 125°C
(Multiple Choice)
4.8/5  (30)
(30)
Copper crystallizes in a face-centered cubic unit cell. The density of copper is 8.94 g/cm3. Calculate the length of the edge of the unit cell in pm.
(Short Answer)
4.8/5  (39)
(39)
Which of the following substances would have the highest critical temperature?
(Multiple Choice)
4.9/5  (39)
(39)
The molar enthalpy of vaporization of boron tribromide is 30.5 kJ/mol, and its normal boiling point is 91°C. What is the vapor pressure of BBr3 at 20°C?
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.9/5  (43)
(43)
The molecular property related to the ease with which the electron density in a neutral atom or molecule can be distorted is called
(Multiple Choice)
4.7/5  (32)
(32)
The structural form of the element Ge closely resembles the structure of
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)