Exam 14: Aqueous Equilibria: Acids and Bases
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The value of Ka for a 0.250 M HCN solution having a pH of 4.956 is ________.
(Short Answer)
4.8/5  (38)
(38)
Which one of the following is least able to behave as a Lewis base?
(Multiple Choice)
4.8/5  (40)
(40)
A solution with a hydroxide ion concentration of 4.15 × 10- 6 M is ________ and has a hydrogen ion concentration of ________.
(Multiple Choice)
4.9/5  (35)
(35)
What is the pH of a 0.10 M H2Se solution that has the stepwise dissociation constants
Ka1 = 1.3 × 10-4 and Ka2 = 1.0 × 10-11?
(Multiple Choice)
4.9/5  (36)
(36)
Acetic acid CH3COOH,has an acid dissociation constant of 1.8 × 10-5.What is the conjugate base of acetic acid and what is its base dissociation constant?
(Multiple Choice)
4.9/5  (28)
(28)
A 0.050 M solution of hydroxylamine,NH2OH,having Kb = 9.1 × 10-9 has a pH of ________.
(Short Answer)
4.8/5  (35)
(35)
The following pictures represent solutions of three salts MA;water molecules have been omitted for clarity.Dotted spheres represent Ay- ions;gray spheres represent Mx+ ions;black spheres represent oxygen atoms;and unshaded spheres represent hydrogen atoms. 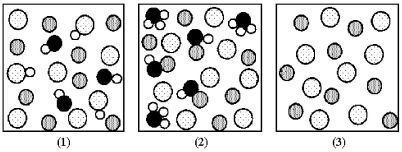 -Which picture represents an acidic salt?
-Which picture represents an acidic salt?
(Multiple Choice)
4.9/5  (35)
(35)
How many grams of pyridine are there in 100 mL of an aqueous solution that has a pH of 9.00? The Kb for pyridine is 1.9 × 10-9 and the equation of interest is
C5H5N(aq)+ H2O(l)⇌ C5H5NH+(aq)+ OH-(aq).
(Multiple Choice)
4.9/5  (33)
(33)
Which one of the following is not considered to be a Lewis base?
(Multiple Choice)
4.9/5  (39)
(39)
Phenobarbital is an antiepileptic drug with a water solubility of 4.3 × 10-3 M and pKa = 7.4.What is the pH and percent ionization of 4.3 × 10-3 M phenobarbital?
(Short Answer)
4.9/5  (32)
(32)
Calculate the pH of a 0.020 M carbonic acid solution,H2CO3(aq),that has the stepwise dissociation constants Ka1 = 4.3 × 10-7 and Ka2 = 5.6 × 10-11.
(Multiple Choice)
4.7/5  (45)
(45)
Write a balanced equation for the dissociation of the Br∅nsted-Lowry acid HSO4- in water.
(Multiple Choice)
4.8/5  (31)
(31)
What is the hydroxide ion concentration of a lye solution that has a pH of 11.20?
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the pH of a 1.60 M CH3NH3Cl solution.Kb for methylamine,CH3NH2,is 3.7 × 10-4.
(Multiple Choice)
5.0/5  (33)
(33)
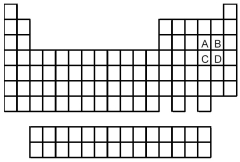 -Of the elements indicated on the periodic table shown above,which forms the weakest binary acid,H2X or HX,where X = A,B,C,or D?
-Of the elements indicated on the periodic table shown above,which forms the weakest binary acid,H2X or HX,where X = A,B,C,or D?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 21 - 40 of 224
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)