Exam 15: Applications of Aqueous Equilibria
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
What is the magnitude of the change in pH when 0.005 moles of HCl is added to 0.100 L of a buffer solution that is 0.100 M in CH3CO2H and 0.100 M NaCH3CO2? The Ka for acetic acid is1.8 × 10-5.
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
C
The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 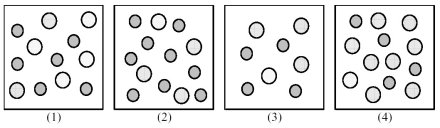 -If solution (1)is a saturated solution of AgCl,which of solutions (1)-(4)represents the solution after a small amount of HNO3 is added and equilibrium is restored?
-If solution (1)is a saturated solution of AgCl,which of solutions (1)-(4)represents the solution after a small amount of HNO3 is added and equilibrium is restored?
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
A
Selenous acid,H2SeO3 has acid dissociation constants Ka1 = 3.5 × 10-2 and Ka2 = 5 × 10-8.When 25.00 mL of 0.100 M selenous acid is titrated with 0.200 M NaOH the first equivalence point occurs at pH = ________.
Free
(Short Answer)
5.0/5  (44)
(44)
Correct Answer:
4.2
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 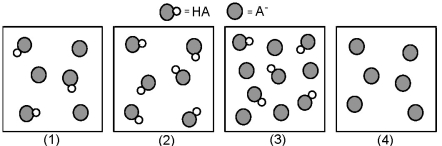 -Which solution has the greatest buffer capacity?
-Which solution has the greatest buffer capacity?
(Multiple Choice)
4.7/5  (31)
(31)
The half equivalence point in the titration of 0.100 M CH3NH2 (Kb = 3.7 × 10-4)with 0.250 M HCl occurs at pH = ________.
(Short Answer)
4.8/5  (26)
(26)
Precipitation of an ionic compound will occur upon mixing of desired reagents if the initial ion product is ________.
(Multiple Choice)
4.9/5  (33)
(33)
What is the pH of a buffer system prepared by dissolving 10.70 grams of NH4Cl and 25.00 mL of
12 M NH3 in enough water to make 1.000 L of solution? Kb = 1.80 × 10-5 for NH3.
(Multiple Choice)
4.8/5  (39)
(39)
In which of the following solutions would solid PbCl2 be expected to be the least soluble at 25°C?
(Multiple Choice)
4.9/5  (32)
(32)
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN)2- is 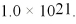
(Multiple Choice)
4.8/5  (38)
(38)
A buffer solution is prepared by dissolving 27.22 g of KH2PO4 and 3.37 g of KOH in enough water to make 0.100 L of solution.What is the pH of the H2PO4-/HPO42- buffer if the Ka2 = 6.2 × 10-8?
(Multiple Choice)
4.9/5  (46)
(46)
The balanced net ionic equation for the neutralization reaction involving equal molar amounts of HNO3 and KOH is ________.
(Short Answer)
4.9/5  (35)
(35)
What volume of 0.100 M NaOH is needed to make 100.0 mL of a buffer solution with a pH of 6.00 if one starts with 50.0 mL of 0.100 M potassium hydrogen phthalate? The Ka2 for potassium hydrogen phthalate is 3.1 × 10-6.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following combinations of chemicals could be used to make a buffer solution?
(Multiple Choice)
4.8/5  (43)
(43)
The pH of a 0.150 M formic acid/0.250 M sodium formate buffer = ________? The Ka of formic acid is 1.8 × 10-4.
(Short Answer)
4.8/5  (37)
(37)
What is the percent dissociation of acetic acid if the solution has a pH = 4.74 and a pKa = 4.74?
(Multiple Choice)
4.9/5  (44)
(44)
What is the pH of the solution formed when 25 mL of 0.173 M NaOH is added to 35 mL of 0.342 M HCl?
(Short Answer)
4.9/5  (33)
(33)
Which of these neutralization reactions has a pH = 7 when equal molar amounts of acid and base are mixed?
(Multiple Choice)
4.8/5  (35)
(35)
The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 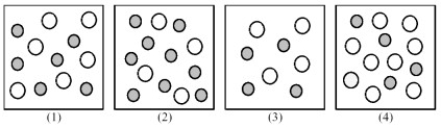 -If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of NaOH is added and equilibrium is restored?
-If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of NaOH is added and equilibrium is restored?
(Multiple Choice)
4.8/5  (37)
(37)
The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions.  -If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
-If solution (1)is a saturated solution of CaCO3,which of solutions (1)-(4)represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the solubility (in g/L)of silver chromate in water at 25°C if the Ksp for Ag2CrO4 is
1)1 × 10-12.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)