Exam 5: Periodicity and the Atomic Structure of Atoms
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Which of the following is not quantized?
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
B
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 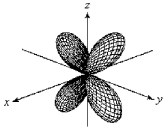
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
C
What is the general valence-electron ground-state electron configuration for neutral alkaline earth metals?
(Multiple Choice)
4.9/5  (32)
(32)
The number of orbitals having the quantum numbers,n = 4 and l = 2 is ________.
(Short Answer)
4.8/5  (36)
(36)
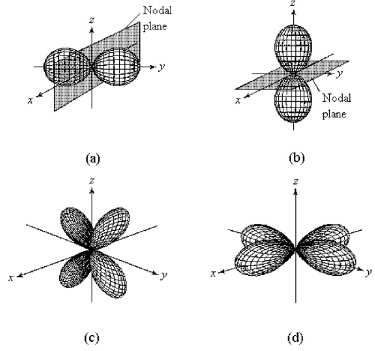 -Which of the above fourth-shell orbitals is a 4dyz orbital?
-Which of the above fourth-shell orbitals is a 4dyz orbital?
(Multiple Choice)
4.7/5  (32)
(32)
Dentists employ light-cured materials to fill cavities.The wavelength of electromagnetic radiation used to photopolymerize restorative materials falls in the ultraviolet or visible region,depending on the instrument employed.Which of these wavelengths is in the UV region?
(Multiple Choice)
4.7/5  (34)
(34)
An oxygen molecule has a mass of 5.3 × 10-26 kg and an approximate diameter of 3.6 × 10-10 m.If the molecule is moving at 400 m/s (1000 mph)with an uncertainty in velocity of 1 m/s,the uncertainty in position
(Multiple Choice)
4.8/5  (34)
(34)
For a multielectron atom,a 3s orbital lies lower in energy than a 3p orbital because
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following have the same number of valence electrons?
(Multiple Choice)
4.9/5  (32)
(32)
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order). 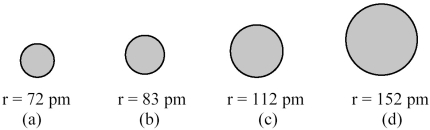 -Which one of these spheres represents an atom of Li?
-Which one of these spheres represents an atom of Li?
(Multiple Choice)
4.7/5  (39)
(39)
According to the Balmer-Rydberg equation,transitions from n = 5 to m = 2 result in a photons of light that give rise to a spectral line with what color?
(Short Answer)
4.8/5  (41)
(41)
Which of the following is not a valid set of quantum numbers?
(Multiple Choice)
4.8/5  (33)
(33)
What are the possible values of n and ml for an electron in a 4dorbital?
(Multiple Choice)
5.0/5  (29)
(29)
Which orbital-filling diagram represents the ground state of oxygen?
(Multiple Choice)
4.8/5  (36)
(36)
What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg)moving at a velocity of
3)0 × 107 m/s (10% of the speed of light)?
(Multiple Choice)
4.8/5  (39)
(39)
The algebraic signs (+ and -)sometimes written within the lobes of orbitals are analogous to different ________ of a wave.
(Short Answer)
4.8/5  (37)
(37)
The ground-state electron configuration of the oxide ion,O2-,is the same as which noble gas?
(Short Answer)
5.0/5  (42)
(42)
Showing 1 - 20 of 158
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)