Exam 19: The Main-Group Elements
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
What statement is not consistent with the chemistry of lead?
(Multiple Choice)
4.8/5  (44)
(44)
The most stable allotrope of sulfur is S8.What are the approximate bond angles in S8?
(Short Answer)
4.7/5  (38)
(38)
Why is H3PO4 a weak triprotic acid whereas H3PO3 is a weak diprotic acid?
(Essay)
4.8/5  (37)
(37)
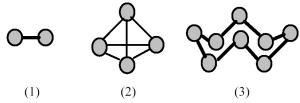
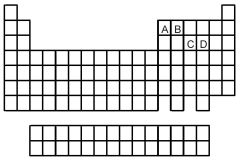
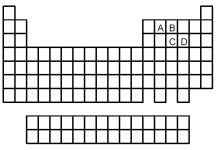 -Which of the elements indicated on the above periodic table forms a common allotrope having molecular structure (2)?
-Which of the elements indicated on the above periodic table forms a common allotrope having molecular structure (2)?
(Multiple Choice)
4.8/5  (43)
(43)
Which M3+ ion of group 3A elements is the easiest to reduce?
(Multiple Choice)
4.8/5  (41)
(41)
What statement is not consistent with the chemistry of tin?
(Multiple Choice)
4.9/5  (33)
(33)
Metallic character for the main group elements generally ________.
(Multiple Choice)
4.9/5  (31)
(31)
Elements in group 3A of the periodic table have the valence electron configuration ________.
(Short Answer)
4.9/5  (37)
(37)
Second row elements differ from heavier elements in all of the following ways except:
(Multiple Choice)
4.8/5  (42)
(42)
The elements indicated by the shaded area in the following periodic table are all 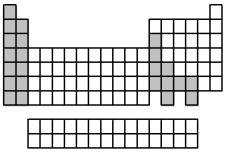
(Multiple Choice)
4.7/5  (34)
(34)
The infinitely extended silicate ion shown in the picture below combines with calcium and magnesium to form the mineral diopside.What is the formula and charge of the repeating unit of the silicate ion? 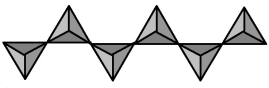
(Multiple Choice)
4.8/5  (34)
(34)
The two-dimensional layer silicate ion shown in the picture below combines with magnesium and hydroxide ions to form talc.What is the formula and charge of the repeating unit of the silicate ion? 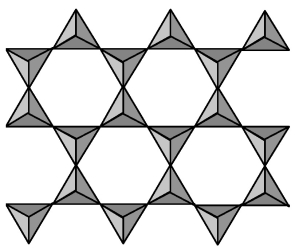
(Multiple Choice)
4.9/5  (35)
(35)
What is not an appropriate method for the isolation of elemental boron?
(Multiple Choice)
4.8/5  (36)
(36)
Which carbon containing compound is an inorganic compound?
(Multiple Choice)
4.9/5  (34)
(34)
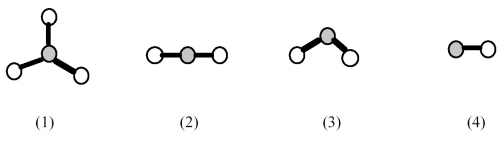
 -Which element indicated on the above periodic table forms a binary oxide with molecular structure (4)shown above?
-Which element indicated on the above periodic table forms a binary oxide with molecular structure (4)shown above?
(Multiple Choice)
4.8/5  (38)
(38)
Given that hypohalous acids form by the following reaction
X2(aq)+ H2O(l)⇌ HOX(aq)+ H+(aq)+ X-(aq)
Which of the following changes will increase the yield of HOX?
(Multiple Choice)
4.7/5  (42)
(42)
Compared to the terminal B-H bonds the bridging H-B bonds in B2H6 are
(Multiple Choice)
4.8/5  (37)
(37)
Using principles discussed in chapters 15 and 19,determine which of the following is the strongest acid.
(Multiple Choice)
4.8/5  (30)
(30)
Showing 181 - 200 of 202
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)