Exam 19: The Main-Group Elements
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
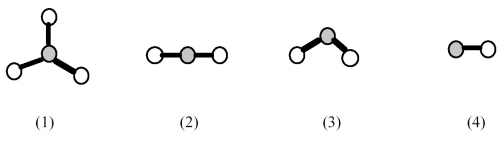
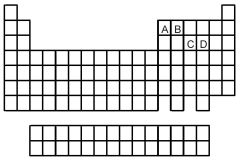 -Which element indicated on the above periodic table forms a binary oxide with molecular structure (1)shown above?
-Which element indicated on the above periodic table forms a binary oxide with molecular structure (1)shown above?
(Multiple Choice)
4.9/5  (40)
(40)
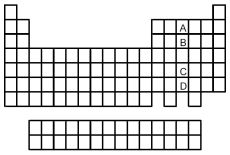 -Which of the elements indicated on the above periodic table is the group 5A element with the smallest atomic radius?
-Which of the elements indicated on the above periodic table is the group 5A element with the smallest atomic radius?
(Multiple Choice)
4.8/5  (28)
(28)
Which one of the following elements forms the most acidic binary oxide?
(Multiple Choice)
4.7/5  (34)
(34)
At 25°C the elements indicated by the shaded area in the following periodic table are all 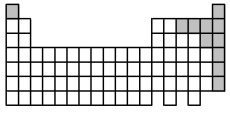
(Multiple Choice)
4.8/5  (41)
(41)
What is the geometric structure of BF3 and the hybridization of the B atom?
(Multiple Choice)
4.9/5  (34)
(34)
What are the basic oxides of the following set Na2O,MgO,P4O10,SO3,and Cl2O7?
(Multiple Choice)
4.7/5  (37)
(37)
The elements indicated by the shaded area in the following periodic table are all 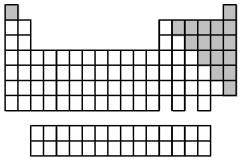
(Multiple Choice)
4.8/5  (33)
(33)
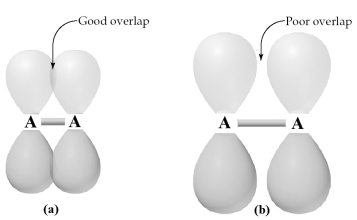 -Which of these molecules is most likely to contain good π overlap of p orbitals,as shown in figure (a),rather than poor π overlap of p orbitals,as shown in figure (b)?
-Which of these molecules is most likely to contain good π overlap of p orbitals,as shown in figure (a),rather than poor π overlap of p orbitals,as shown in figure (b)?
(Multiple Choice)
5.0/5  (36)
(36)
Shown below are four oxoanions of sulfur.Small gray spheres represent hydrogen,unshaded spheres represent oxygen,and dotted spheres represent sulfur. 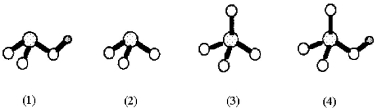 -Which oxoanion is the strongest acid?
-Which oxoanion is the strongest acid?
(Multiple Choice)
4.8/5  (30)
(30)
Wollastonite,CaSiO3,is an example of a(n)________ (orthosilicate,single-strand silicate,double-strand silicate,and cyclic silicate).
(Short Answer)
4.8/5  (30)
(30)
The percentage of carbon in the endohedral fullerene complexes KrC60 and XeC60 is ________%C and ________%C,respectively.
(Short Answer)
5.0/5  (39)
(39)
What statement about nitrogen is not consistent with its chemistry?
(Multiple Choice)
4.8/5  (39)
(39)
What is not an appropriate method of making phosphoric acid?
(Multiple Choice)
4.8/5  (37)
(37)
What is an appropriate method for the synthesis of phosphorus?
(Multiple Choice)
4.9/5  (42)
(42)
What statement is not representative of the chemistry of H2S?
(Multiple Choice)
5.0/5  (38)
(38)
Elements in period 2 of the periodic table differ markedly from the heavier elements in the same group due to their especially small ________ and high ________.
(Short Answer)
4.9/5  (41)
(41)
Mica cleaves into thin sheets because at the molecular level its structure is ________.
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 40 of 202
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)