Exam 11: Carbonyl Compounds and Redox Reactions
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
Vitamin K (whose simplified structure is given below) is required for blood clotting. One of the reactions involved in blood clotting is shown below. This reaction represents an oxidation. 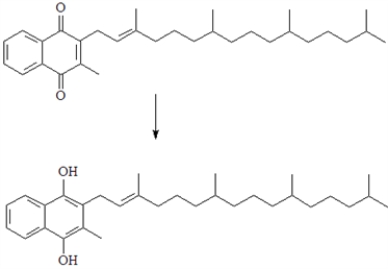
(True/False)
4.8/5  (29)
(29)
How many ketone carbonyl groups are present in the following compound? 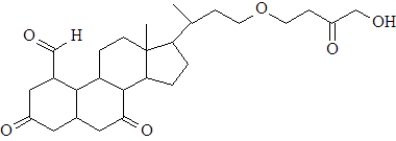
(Multiple Choice)
4.8/5  (37)
(37)
Consider the structures given below. 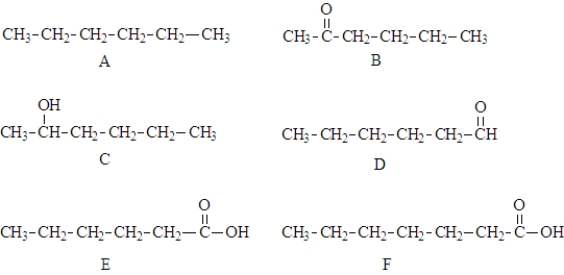 Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _______________________ would be the most soluble in water.
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _______________________ would be the most soluble in water.
(Short Answer)
4.8/5  (37)
(37)
Consider the structures given below.  Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure_______________ could be oxidized to structure ____________________.
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure_______________ could be oxidized to structure ____________________.
(Short Answer)
4.8/5  (30)
(30)
Consider the structures given below.  Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ could serve as both a hydrogen bond donor and a hydrogen bond acceptor.
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ could serve as both a hydrogen bond donor and a hydrogen bond acceptor.
(Short Answer)
4.8/5  (31)
(31)
Consider the structures given below.  Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ would produce an aqueous solution with a pH less than 7..
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ would produce an aqueous solution with a pH less than 7..
(Short Answer)
4.8/5  (33)
(33)
Which of the following types of organic compounds contains a carbonyl group?
(Multiple Choice)
4.8/5  (39)
(39)
If 2-hexanol underwent dehydration followed by dehydrogenation, the final product would be:
(Multiple Choice)
4.8/5  (41)
(41)
Name the reactant molecule shown below and then draw the product of the dehyrogenation of this substance. The affected part of the molecule is enclosed in the oval. 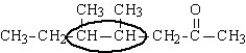
(Essay)
4.8/5  (26)
(26)
If the following compound was oxidized as much as possible, CH3CH2CH2CH2CH2CH2CH2CH2OH
Which of the following would be the product of the complete reaction?
(Multiple Choice)
4.7/5  (38)
(38)
Consider the structures given below.  Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-With like molecules, structure _______________________ could participate in hydrogen bonding.
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-With like molecules, structure _______________________ could participate in hydrogen bonding.
(Short Answer)
4.8/5  (32)
(32)
Consider the structures given below.  Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ would be named hexanal.
Fill in the blanks with the appropriate letter (A,B,C,D,E,F).
-Structure _____________________ would be named hexanal.
(Short Answer)
4.9/5  (28)
(28)
When our bodies need a sudden burst of energy, glucose is converted into carbon dioxide and water.
(True/False)
4.9/5  (35)
(35)
Which of the following products is formed when hydrogen is reacted with 3-methyl-2-butanone under appropriate reaction conditions?
(Multiple Choice)
4.9/5  (37)
(37)
The following is a summary of the steps found in the metabolic pathway known as the Citric Acid Cycle.
Step 1: 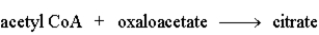 Step 2:
Step 2:  Step 3:
Step 3: 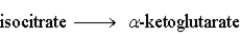 Step 4:
Step 4: 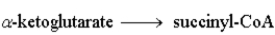 Step 5:
Step 5:  Step 6:
Step 6:  Step 7:
Step 7:  Step 8:
Step 8: 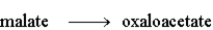 Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-The reactant of step 5 is _______________________.
Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-The reactant of step 5 is _______________________.
(Short Answer)
4.9/5  (42)
(42)
The following is a summary of the steps found in the metabolic pathway known as the Citric Acid Cycle.
Step 1: 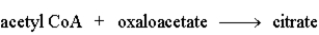 Step 2:
Step 2:  Step 3:
Step 3: 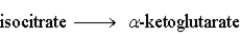 Step 4:
Step 4: 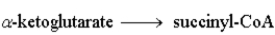 Step 5:
Step 5:  Step 6:
Step 6:  Step 7:
Step 7:  Step 8:
Step 8: 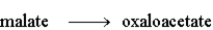 Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-Step 7 converts -CH-OH to -C=O. This reaction _____________________ energy.
Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-Step 7 converts -CH-OH to -C=O. This reaction _____________________ energy.
(Short Answer)
4.8/5  (43)
(43)
The following is a summary of the steps found in the metabolic pathway known as the Citric Acid Cycle.
Step 1: 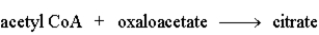 Step 2:
Step 2:  Step 3:
Step 3: 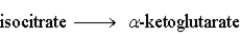 Step 4:
Step 4: 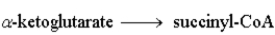 Step 5:
Step 5:  Step 6:
Step 6:  Step 7:
Step 7:  Step 8:
Step 8: 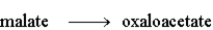 Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-Step 6 converts the -CH2-CH2- of succinate to the -CH=CH- of fumarate. This reaction would be classified as a(n) ______________________ reaction.
Fill in the blanks in the following question with the appropriate term from the list below.
oxaloacetate oxidation
citrate reduction
isocitrate consumes
a-ketoglutarate produces
succinyl-CoA NADH
succinate FAD
fumarate NADPH
malate
-Step 6 converts the -CH2-CH2- of succinate to the -CH=CH- of fumarate. This reaction would be classified as a(n) ______________________ reaction.
(Short Answer)
4.9/5  (35)
(35)
Showing 41 - 60 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)