Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
What is the minimum mass of sulfur dioxide necessary to produce 200.g of sulfuric acid in the following reaction?
2SO2 + O2 + 2H2O 2H2SO4
(Short Answer)
4.8/5  (43)
(43)
Calculate the volume of 0.15 mole of Br2.The density of Br2 is 3.12 g/mL.
(Short Answer)
4.8/5  (29)
(29)
Oxidation of a hydrocarbon gave a product composed of carbon, hydrogen, and oxygen.The product that was purified and sent off for elemental analysis giving the following mass percents: 68.85% C and 4.95% H.Determine the empirical formula of this compound.
(Short Answer)
4.8/5  (38)
(38)
There are two stable isotopes of chlorine: chlorine-35, with a mass of 34.968853 amu; and chlorine-37, with a mass of 36.965903.Given that the average atomic mass of a chlorine atom is 35.45 amu, which of the following statements is true?
(Multiple Choice)
4.8/5  (40)
(40)
Which one of the following does not represent 1.000 mol of the indicated substance?
(Multiple Choice)
4.9/5  (29)
(29)
Ferrocene, Fe(C5H5)2(s), can be prepared by reacting 3.0 g of FeCl2(s)with an equal mass of cyclopentadiene, C5H6(l), and an excess of KOH, as shown in the following reaction
FeCl2 + 2C5H6 + 2KOH FeC10H10 + 2H2O
A.What is the limiting reagent in this procedure?
B.Based on your answer to part A, what mass of Fe(C5H5)2 could theoretically be formed?
C.A student who carried out this reaction obtained 2.7 g of ferrocene.What was the percent yield for this reaction?
(Essay)
4.8/5  (38)
(38)
A chemistry student determined the empirical formula for titanium sulfide (TixSy).To do so, she reacted titanium with excess sulfur in a crucible, and recorded the following data: 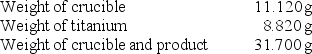 What is the empirical formula of titanium sulfide?
What is the empirical formula of titanium sulfide?
(Short Answer)
4.8/5  (29)
(29)
Determine the number of moles of water produced by the reaction of 155 g of ammonia and 356 g of oxygen.
4NH3 + 5O2 4NO + 6H2O
(Short Answer)
4.7/5  (39)
(39)
How many moles of HCl are represented by 1.0 × 1019 HCl molecules?
(Multiple Choice)
4.8/5  (32)
(32)
What is the coefficient of H2SO4 when the following equation is properly balanced?
___ Ca3(PO4)2 + ___ H2SO4 ___ CaSO4 + ___ H3PO4
(Multiple Choice)
4.8/5  (45)
(45)
Vanadium(V)oxide reacts with calcium according to the chemical equation below.When 10.0 moles of V2O5 are mixed with 10.0 moles of Ca, which is the limiting reagent?
V2O5(s)+ 5Ca(l) 2V(l)+ 5CaO(s)
(Multiple Choice)
5.0/5  (34)
(34)
Showing 181 - 194 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)