Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
What is the theoretical yield of chromium that can be produced by the reaction of 40.0 g of Cr2O3 with 8.00 g of aluminum according to the chemical equation below? 2Al + Cr2O3 Al2O3 + 2Cr
(Multiple Choice)
4.9/5  (46)
(46)
A compound with a percent composition by mass of 24.61% C, 2.75% H, and 72.64% Cl has a molar mass of 292.82 g/mol.What is the empirical formula of the compound?
(Short Answer)
4.9/5  (24)
(24)
Calculate the average atomic mass of silver using the following data: 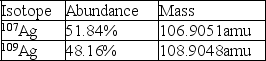
(Multiple Choice)
4.8/5  (44)
(44)
What is the coefficient of H2O when the following equation is properly balanced? ___ Al4C3 + ___ H2O ___ Al(OH)3 + ___ CH4
(Multiple Choice)
4.8/5  (37)
(37)
Balance the following chemical equation:
Al(s)+ Co(NO3)2(aq) Al(NO3)3(aq)+ Co(s)
(Essay)
4.8/5  (34)
(34)
If 0.274 moles of a substance weighs 62.5 g, what is the molar mass of the substance, in units of g/mol?
(Multiple Choice)
4.9/5  (47)
(47)
Ammonia reacts with oxygen to form nitric oxide and water vapor:
4NH3 + 5O2 4NO + 6H2O
What is the theoretical yield of water, in moles, when 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react?
(Multiple Choice)
4.9/5  (37)
(37)
The percent composition by mass of an unknown chlorinated hydrocarbon was found to be 37.83% C, 6.35% H, and 55.83% Cl by mass.What is the empirical formula of this compound?
(Multiple Choice)
4.8/5  (32)
(32)
How many moles of iron are present in an iron cylinder that weighs 25 g?
(Short Answer)
4.9/5  (43)
(43)
Which of the following samples contains the greatest number of atoms?
(Multiple Choice)
4.8/5  (42)
(42)
The mineral pyrolusite is a compound of 55Mn and 16O.If 63% of the mass of pyrolusite is due to manganese, what is the empirical formula of pyrolusite?
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the percent composition by mass of oxygen in Na2CO3.
(Short Answer)
4.8/5  (32)
(32)
Acetylene gas, HCCH(g), can be generated in the laboratory by adding calcium carbide to excess water, as shown in the following reaction
CaC2(s)+ H2O(l) HCCH(g)+ CaO(s)
How many grams of CaC2 would be required to generate 0.20 moles of HCCH(g)?
(Short Answer)
4.9/5  (42)
(42)
Calculate the mass of FeS formed when 9.42 g of iron reacts with 8.50 g of sulfur according to the following reaction.
Fe(s)+ S(s) FeS(s)
(Multiple Choice)
4.9/5  (42)
(42)
A 0.600 g sample of a compound of arsenic and oxygen was found to contain 0.454 g of arsenic.What is the empirical formula of the compound?
(Short Answer)
4.9/5  (38)
(38)
Calculate the average atomic mass of lithium using the following data: 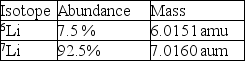
(Multiple Choice)
4.8/5  (32)
(32)
What is the coefficient of H2O when the equation below is properly balanced? ___ PCl3(l)+ ___ H2O(l) ___ H3PO3(aq) + ___ HCl(aq)
(Multiple Choice)
4.9/5  (36)
(36)
Showing 121 - 140 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)