Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
Sulfur dioxide gas reacts with oxygen gas and water according to the chemical reaction below.When 4.5 g of SO2 are mixed with excess O2 and H2O, how many grams of H2SO4 are produced? 2SO2(g)+ O2(g)+ 2H2O(l) 2H2SO4(l)
(Multiple Choice)
4.8/5  (46)
(46)
An atom of bromine has a mass about four times greater than that of an atom of neon.How many grams of neon will contain the same number of atoms as 1,000 g of bromine?
(Multiple Choice)
4.7/5  (35)
(35)
Pressurized metal gas cylinders are generally used to store commonly used gases in the laboratory.At times it can be easier to chemically prepare occasionally used gases..For example, nitrogen monoxide, NO(g), can be prepared in the lab using the following chemical reaction:
3Cu(s)+ 8HNO3(aq) 2NO(g)+ 3Cu(NO3)2(aq)+ 4H2O(l)
If 5.0 g of copper metal was added to an aqueous solution containing 2.5 moles of HNO3, how many moles of NO(g)would be produced, assuming a 100% yield.
(Short Answer)
4.8/5  (38)
(38)
A compound with an empirical formula of C2H4Br has a molar mass of 215.90 g/mol.What is the molecular formula?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following CO2 samples contains the greatest number of moles of CO2?
(Multiple Choice)
4.9/5  (39)
(39)
Aluminum hydroxide reacts with nitric acid to form aluminum nitrate and water.What mass of water can be formed by the reaction of 15.0 g of aluminum hydroxide with excess nitric acid?
(Multiple Choice)
4.9/5  (37)
(37)
The first step in the Ostwald process for producing nitric acid is
4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g).
If the reaction of 150.g of ammonia with 150.g of oxygen gas yields 87.g of nitric oxide (NO), what is the percent yield of this reaction?
(Multiple Choice)
4.9/5  (42)
(42)
Which one of the following is an example of a balanced chemical reaction?
(Multiple Choice)
4.9/5  (36)
(36)
A mass spectrometer works by ionizing atoms or molecules, and then accelerating them through oppositely charged plates.The mass is obtained by
(Multiple Choice)
4.8/5  (28)
(28)
Calculate the mass of excess reagent remaining at the end of the reaction in which 90.0 g of SO2 are mixed with 100.0 g of O2. 2SO2 + O2 2SO3
(Multiple Choice)
4.8/5  (39)
(39)
If 0.66 mole of a substance has a mass of 99 g, what is the molecular mass of the substance?
(Short Answer)
4.7/5  (36)
(36)
Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 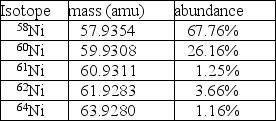 A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
(Essay)
4.8/5  (28)
(28)
The molecular formula of aspirin is C9H8O4.How many aspirin molecules are present in one 500-milligram tablet?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following NH3 samples contains the greatest number of moles of NH3?
(Multiple Choice)
4.8/5  (37)
(37)
The reaction of 44.1 g of Cr2O3 with 35.0 g of Al produced 25.6 g of Cr.What is the percent yield for this reaction? 2Al + Cr2O3 Al2O3 + 2Cr
(Multiple Choice)
4.8/5  (32)
(32)
A compound with a percent composition by mass of 87.5% N and 12.5% H was recently discovered.What is the empirical formula for this compound?
(Short Answer)
4.8/5  (32)
(32)
Showing 41 - 60 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)