Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
A sample of iron metal is placed in a graduated cylinder.It is noted that 10.4 mL of water is displaced by the iron.The iron is then reacted with excess hydrochloric acid to produce iron (II)chloride and hydrogen gas.Given the density for iron is 7.86 g/mL, how many grams of iron (II)chloride are produced in the reaction?
Fe(s)+ 2HCl(aq) FeCl2(aq)+ H2(g)
(Multiple Choice)
4.7/5  (32)
(32)
A compound was discovered whose composition by mass is 85.6% C and 14.4% H.Which of the following could be the molecular formula of this compound?
(Multiple Choice)
4.8/5  (43)
(43)
An average atom of uranium (U)is approximately how many times heavier than an atom of potassium?
(Multiple Choice)
4.9/5  (36)
(36)
Refer to the (unbalanced)equation CS2 + CaO CO2 + CaS.How many grams of CaO are required to react completely with 38 g of CS2?
(Short Answer)
4.9/5  (39)
(39)
A silver wire has a diameter of 0.500 mm.What length of this wire contains exactly 1.00 mol of silver? (density of Ag = 10.5 g/cm3)
(Multiple Choice)
4.8/5  (31)
(31)
Lithium metal reacts with nitrogen gas to form lithium nitride.Identify the balanced reaction that describes this process.
(Multiple Choice)
4.8/5  (41)
(41)
A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 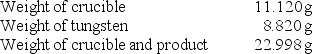 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
(Short Answer)
4.7/5  (35)
(35)
Formaldehyde has the formula CH2O.How many molecules are there in 0.11 g of formaldehyde?
(Multiple Choice)
4.8/5  (35)
(35)
What is the coefficient for O2 when the following combustion reaction of a hydrocarbon is balanced?
___ C7H14 + ___ O2 ___ CO2 + ___ H2O
(Multiple Choice)
4.9/5  (44)
(44)
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mole of V2O5 with 4.0 moles of calcium based on the following chemical reaction? V2O5(s)+ 5Ca(l) 2V(l)+ 5CaO(s)
(Multiple Choice)
4.8/5  (37)
(37)
Hydrogen chloride gas can be prepared by the following reaction:
2NaCl(s)+ H2SO4(aq) 2HCl(g)+ Na2SO4(s)
How many grams of HCl can be prepared from 2.00 mol H2SO4 and 2.56 mol NaCl?
(Multiple Choice)
4.8/5  (32)
(32)
Phosphorus reacts with iodine as shown in the chemical reaction below.What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus?
2P(s)+ 3I2(s) 2PI3(s)
(Short Answer)
4.8/5  (44)
(44)
Phosphorus pentachloride reacts with water to form hydrochloric acid and phosphoric acid.How many total moles of acid are formed when starting with 4.5 g of PCl5 and excess H2O? PCl5 + 4H2O 5HCl + H3PO4
(Multiple Choice)
4.8/5  (46)
(46)
When balanced the coefficient of O2 in the following equation is
__ C2H4 + __ O2 __ CO2 + __ H2O
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following is an example of a balanced chemical reaction?
(Multiple Choice)
4.9/5  (30)
(30)
Boron obtained from borax deposits in Death Valley consists of two isotopes.They are boron-10 and boron-11 with atomic masses of 10.013 amu and 11.009 amu, respectively.The atomic mass of boron is 10.81 amu (see periodic table).Which isotope of boron is more abundant, boron-10 or boron-11?
(Multiple Choice)
5.0/5  (46)
(46)
Showing 141 - 160 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)