Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 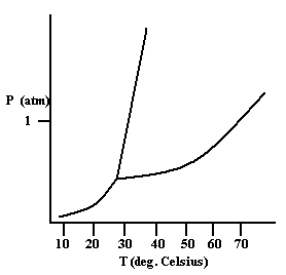
(Multiple Choice)
4.8/5  (32)
(32)
Find the temperature at which water boils on a day in the mountains when the barometric pressure is 593 mmHg.(Given: the heat of vaporization of water is 40.79 kJ/mol)
(Multiple Choice)
4.8/5  (43)
(43)
Iron crystallizes in a body-centered cubic unit.The edge of this cell is 287 pm.Calculate the density of iron.
(Short Answer)
4.8/5  (26)
(26)
Osmium tetroxide, OsO4, is a soft crystal that melts at 40°C.The liquid does not conduct electricity.What kind of crystal is this?
(Short Answer)
4.9/5  (36)
(36)
Crystals of elemental sulfur are easily crushed, and melt at 113°C.Liquid sulfur does not conduct electricity.What kind of crystal is this?
(Short Answer)
4.8/5  (37)
(37)
The triple point of iodine is at 0.12 atm and 115°C.Thus, liquid I2
(Multiple Choice)
4.8/5  (35)
(35)
The molar enthalpy of vaporization of hexane (C6H14)is 28.9 kJ/mol, and its normal boiling point is 68.73°C.What is the vapor pressure of hexane at 25°C?
(Multiple Choice)
4.8/5  (30)
(30)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 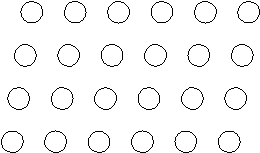 On the drawing above, sketch the unit cell of this crystal.
On the drawing above, sketch the unit cell of this crystal.
(Essay)
4.9/5  (44)
(44)
Calculate the amount of enthalpy required to heat 25.0 g of solid benzene (C6H6)at -10°C to liquid benzene at 20.0°C.Thermodynamic data for benzene: specific heat of solid benzene = 1.52 J/g·°C; specific heat of liquid benzene = 1.73 J/g·°C; melting point = 5.5°C; Hfus = 9.9 kJ/mol.
(Short Answer)
4.8/5  (42)
(42)
The intermolecular forces present in HSCH2CH2SH include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
(Multiple Choice)
4.8/5  (42)
(42)
A face-centered cubic unit cell is the repeating unit in which type of crystal packing?
(Multiple Choice)
4.9/5  (33)
(33)
Magnesium oxide, MgO, melts at 2,800°C and is very hard.The liquid conducts electricity very well.What kind of crystal is this?
(Short Answer)
4.9/5  (27)
(27)
Which property of water allows a razor blade to float on it without sinking?
(Multiple Choice)
4.8/5  (36)
(36)
The intermolecular forces present in CH3NH2 include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
(Multiple Choice)
4.8/5  (27)
(27)
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure.The edge length of this cubic unit cell is 540.9 pm.Determine the density of zincblende.
(Multiple Choice)
4.8/5  (37)
(37)
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 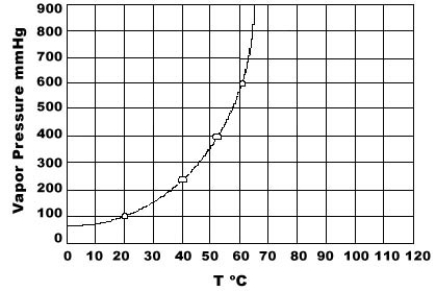
(Multiple Choice)
4.9/5  (44)
(44)
Showing 61 - 80 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)