Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
The number of nearest neighbors (atoms that make contact)around each atom in a face-centered cubic lattice of a metal is
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
(Multiple Choice)
4.9/5  (32)
(32)
The structural form of the element Ge closely resembles the structure of
(Multiple Choice)
4.8/5  (33)
(33)
The freezing point of a liquid does not change as the atmospheric pressure changes.
(True/False)
4.8/5  (30)
(30)
For which of the following species are the dispersion forces strongest?
(Multiple Choice)
4.9/5  (43)
(43)
Identify the dominant (strongest)type of intermolecular force present in RbCl(s).
(Short Answer)
4.8/5  (42)
(42)
In the following picture, each arrow represents a molecule or atom.Based on the arrangement in the solid state as shown, which of the following best represents the unit cell? 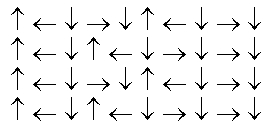
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following properties is not influenced by hydrogen bonding?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following substances is expected to have the highest molar heat of vaporization ( Hvap)?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 141 - 149 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)