Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
Methane has a heat of fusion of 0.84 kJ/mol and a heat of vaporization of 9.2 kJ/mol.Estimate the value for the heat of sublimation.
(Short Answer)
4.7/5  (37)
(37)
The mineral manganosite, manganese(II)oxide, crystallizes in the rock salt structure (the face-centered structure adopted by NaCl)with a density of 5.365 g/cm3.Find the unit cell edge length of manganosite.
(Multiple Choice)
5.0/5  (32)
(32)
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure).How many Cl- ions are present in one unit cell of this crystal?
(Multiple Choice)
4.8/5  (31)
(31)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 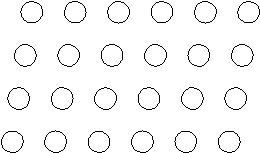 How many atoms are in one unit cell?
How many atoms are in one unit cell?
(Essay)
4.8/5  (39)
(39)
Octane, C8H18, boils at 125°C as compared to water, which boils at 100°C.This information suggests that the dispersion forces in nonpolar octane molecules are stronger than dispersion forces and hydrogen bonding in water.
(True/False)
4.9/5  (41)
(41)
Palladium crystallizes in a face-centered cubic unit cell.Its density is 12.0 g/cm3 at 27°C.Calculate the atomic radius of Pd.
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the amount of heat needed to melt 2.00 kg of iron at its melting point (1,809 K), given that Hfus = 13.80 kJ/mol.
(Multiple Choice)
4.8/5  (29)
(29)
Which one of the following crystallizes in a metallic lattice?
(Multiple Choice)
4.9/5  (42)
(42)
Ethanol and dimethyl ether have the same molecular formula, C2H6O.Ethanol boils at 78.4°C.Dimethyl ether boils at -23.7°C.Their structural formulas are, respectively, CH3CH2OH and CH3OCH3.Explain why the boiling point of the ether is so much lower than the boiling point of ethanol.
(Essay)
4.9/5  (33)
(33)
Octane is a liquid component of gasoline.Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg. 400 mmHg @ 104°C, 500 mmHg @ 111°C, 600 mmHg @ 117°C, 700 mmHg @ 122°C, 760 mmHg @ 125°C
(Multiple Choice)
4.9/5  (34)
(34)
Which of following can form hydrogen bonds with water molecules? (1)Na+ (2)CH3COOH (3)C2H6 (4)CH3NH2
(Multiple Choice)
5.0/5  (39)
(39)
The molar enthalpy of vaporization of boron tribromide is 30.5 kJ/mol, and its normal boiling point is 91°C.What is the vapor pressure of BBr3 at 20°C?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following would be expected to have the lowest vapor pressure at room temperature?
(Multiple Choice)
4.7/5  (44)
(44)
Which is expected to have a higher vapor pressure, C8H18 or C4H10?
(Short Answer)
4.8/5  (38)
(38)
The vapor pressure of ethanol is 400 mmHg at 63.5°C.Its molar heat of vaporization is 39.3 kJ/mol.What is vapor pressure of ethanol, in mmHg, at 34.9°C?
(Multiple Choice)
4.8/5  (35)
(35)
Indicate all the types of intermolecular forces of attraction in CHCl3(l).
(Short Answer)
4.9/5  (27)
(27)
Given that the heat of vaporization of mercury is 59.0 kJ/mol and the vapor pressure of mercury is 0.0017 torr at 25°C, calculate the normal boiling point of mercury.
(Short Answer)
4.8/5  (29)
(29)
Indicate all the types of intermolecular forces of attraction in CH3OH(l).
(Short Answer)
4.9/5  (39)
(39)
Showing 81 - 100 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)