Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
Arrange the following substances in order of increasing boiling point: CH3CH2OH, HOCH2CH2OH, CH3CH2Cl, and ClCH2CH2OH
(Multiple Choice)
4.8/5  (34)
(34)
Identify the dominant (strongest)type of intermolecular force present in Cl2(l).
(Short Answer)
4.9/5  (40)
(40)
All intermolecular forces must be overcome in order for a substance to undergo a phase change from a liquid to a gas
(True/False)
4.9/5  (40)
(40)
The specific heat of liquid ethanol, C2H5OH(l), is 2.46 J/g·°C and the heat of vaporization is 39.3 kJ/mol.The boiling point of ethanol is 78.3 °C.What amount of enthalpy is required to heat 50.0 g of liquid ethanol from 23.0 °C to ethanol vapor at 78.3 °C?
(Multiple Choice)
4.8/5  (40)
(40)
Potassium crystallizes in a body-centered cubic lattice.How many atoms are there per unit cell?
(Multiple Choice)
4.7/5  (34)
(34)
Acetic acid has a heat of fusion of 10.8 kJ/mol and a heat of vaporization of 24.3 kJ/mol.What is the expected value for the heat of sublimation of acetic acid?
(Multiple Choice)
4.7/5  (30)
(30)
Indicate all the types of intermolecular forces of attraction in He(l).
(Short Answer)
4.7/5  (28)
(28)
Sodium iodide, NaI, melts at 651°C.In its liquid state NaI conducts electricity.What kind of crystal is this?
(Short Answer)
4.8/5  (39)
(39)
What mass of water would need to evaporate from your skin in order to dissipate 1.7 × 105 J of heat from your body? H2O(l) H2O(g) Hvap = 40.7 kJ/mol
(Multiple Choice)
4.7/5  (46)
(46)
Identify the dominant (strongest)type of intermolecular force present in H2S(g).
(Short Answer)
4.9/5  (37)
(37)
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C.Given: specific heat (ice)= 2.1 J/g·°C; specific heat (water)= 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
(Multiple Choice)
4.8/5  (27)
(27)
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.8/5  (37)
(37)
How much energy (heat)is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 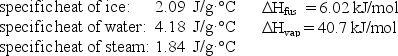
(Multiple Choice)
4.8/5  (37)
(37)
Indicate all the types of intermolecular forces of attraction in HCl(g).
(Short Answer)
4.9/5  (31)
(31)
Which one of the following substances crystallizes as a covalent crystal?
(Multiple Choice)
4.8/5  (37)
(37)
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following constants is/are needed to calculate the amount of energy required to heat 12.0g of H2O(l)at 30.0°C to H2O(l)at 85.0°C? I. Hfus (H2O)
II. Hvap (H2O)
III.specific heat of H2O(s)
IV.specific heat of H2O(l)
V.specific heat of H2O(g)
(Multiple Choice)
4.8/5  (38)
(38)
Showing 41 - 60 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)