Exam 15: Acids and Bases
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
If the pH of pure water is 7.0, what is the hydroxide ion concentration in pure water?
(Short Answer)
4.9/5  (30)
(30)
When 2.0 × 10-2 mole of nicotinic acid (a monoprotic acid)is dissolved in 350.mL of water, the pH is 3.05.What is the Ka of nicotinic acid?
(Short Answer)
4.7/5  (43)
(43)
Which one of these equations represents the reaction of a weak acid with a strong base?
(Multiple Choice)
4.9/5  (44)
(44)
Which one of these net ionic equations represents the reaction of a strong acid with a strong base?
(Multiple Choice)
4.8/5  (36)
(36)
Suppose that ammonia, applied to a field as a fertilizer, is washed into a farm pond containing 3.0 × 106 L of water.If the pH of this pond is found to be 9.81, what volume of liquid ammonia found its way into the pond? [Given: Kb(NH3)= 1.8 × 10-5; the density of liquid ammonia is 0.771 g/cm3]
(Multiple Choice)
4.8/5  (32)
(32)
Al(OH)3 is an amphoteric hydroxide.Write a balanced ionic equation to show its reaction with KOH.
(Essay)
4.8/5  (38)
(38)
The pH of coffee is approximately 5.0.How many times greater is the [H3O+] in coffee than in tap water having a pH of 8.0?
(Multiple Choice)
4.8/5  (43)
(43)
Given the following Kb values, which cation is the strongest acid? 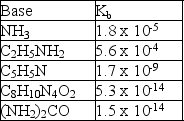
(Multiple Choice)
4.8/5  (37)
(37)
When comparing acid strength of binary acids HX, as X varies within a particular group of the periodic table, which one of these factors dominates in affecting the acid strength?
(Multiple Choice)
4.8/5  (32)
(32)
Morphine, C17H19NO3, is often used to control severe post-operative pain.What is the pH of the solution made by dissolving 25.0 mg of morphine in 100.mL of water? (For morphine, Kb = 1.62 × 10-6.)
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the pH of a 0.15 M solution of HOI (Ka = 2.3 x 10-11)
(Short Answer)
4.8/5  (37)
(37)
Calculate the concentration of malonate ion (C3H2O42-)in a 0.200 M solution of malonic acid (C3H4O4).[For malonic acid, Ka1 = 1.4 × 10-3, Ka2 = 2.0 × 10-6.]
(Multiple Choice)
4.7/5  (39)
(39)
Which one of these salts will form a neutral solution on dissolving in water?
(Multiple Choice)
4.8/5  (34)
(34)
The pH of coffee is approximately 5.0.How many times greater is the [H+] in coffee than in neutral water?
(Multiple Choice)
4.8/5  (39)
(39)
Which one of these salts will form a basic solution upon dissolving in water?
(Multiple Choice)
4.9/5  (42)
(42)
For maleic acid, HOOCCH=CHCOOH, Ka1 = 1.42 × 10-2 and Ka2 = 8.57 × 10-7. What is the concentration of maleate ion (-OOCCH=CHCOO-)in a 0.150 M aqueous solution of maleic acid?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 161 - 178 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)